北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (4): 722-728. doi: 10.19723/j.issn.1671-167X.2024.04.028
血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系
和静1,2,房中则3,杨颖4,刘静5,马文瑶2,霍勇4,高炜6,武阳丰1,2,7,8,谢高强1,2,7,8,*( )
)
- 1. 北京大学第一医院, 北京 100034
2. 北京大学临床医学高等研究院临床研究所, 北京 100191
3. 天津医科大学公共卫生学院, 天津 300070
4. 北京大学第一医院心内科, 北京 100034
5. 首都医科大学附属北京安贞医院临床与流行病研究中心, 北京 100029
6. 北京大学第三医院心内科, 北京 100191
7. 重大疾病流行病学教育部重点实验室(北京大学), 北京 100191
8. 血管稳态与重构全国重点实验室(北京大学), 北京 100191
Relationship between lipid metabolism molecules in plasma and carotid atheroscle-rotic plaques, traditional cardiovascular risk factors, and dietary factors
Jing HE1,2,Zhongze FANG3,Ying YANG4,Jing LIU5,Wenyao MA2,Yong HUO4,Wei GAO6,Yangfeng WU1,2,7,8,Gaoqiang XIE1,2,7,8,*( )
)
- 1. Peking University First Hospital, Beijing 100034, China
2. Clinical Research Institute, Institute of Advanced Clinical Medicine, Peking University, Beijing 100191, China
3. College of Public Health, Tianjin Medical University, Tianjin 300070, China
4. Department of Cardiology, Peking University First Hospital, Beijing 100034, China
5. Center of Clinical and Epidemiology, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China
6. Department of Cardiology, Peking University Third Hospital, Beijing 100191, China
7. Key Laboratory of Epidemiology of Major Diseases (Peking University), Beijing 100191, China
8. State Key Laboratory of Vascular Homeostasis and Remodeling (Peking University), Beijing 100191, China
摘要:
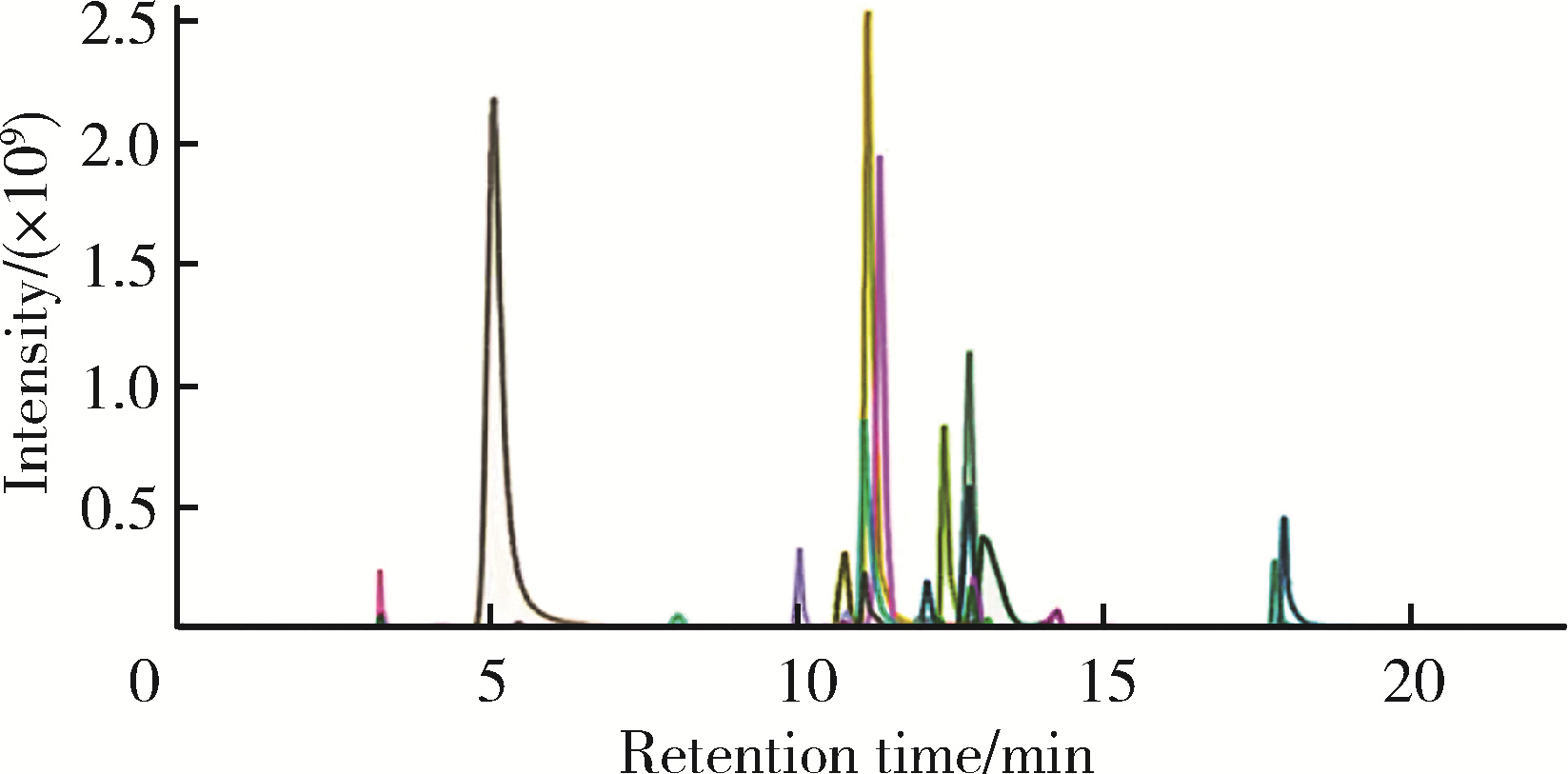
目的: 探索血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素的关系及可能的膳食相关因素。方法: 从参加2012年“亚临床动脉粥样硬化队列10年随访研究”的北京市石景山区1 312名社区人群中,按照入排标准(年龄 < 70岁、无临床心血管病及其他疾病等)筛选出85名有2个以上颈动脉软斑块或混合斑块者,以及相匹配的89名无斑块对照者;然后从中各随机抽取10名分别作为病例组和对照组。颈动脉斑块采用GE Vivid i超声仪(8L探头)确定。采用高效液相色谱-质谱联用法对脂质代谢分子进行检测,检测指标包括113种脂质代谢分子。传统心血管危险因素使用统一的标准问卷进行采集,膳食相关因素采用膳食使用频率和重量问卷进行采集。采用Wilcoxin秩和检验分析病例组和对照组脂质代谢分子的差异,在对照组中,采用Spearman相关法描述有统计学意义的脂质代谢分子与传统心血管危险因素、膳食因素的相关关系。结果: 在113种脂质代谢分子中检测出的53种脂质分子中,C24:0鞘磷脂,C22:0、C24:0神经酰胺,C18:0磷脂酰乙醇胺,C18:2 (Cis) 磷脂酰胆碱,C18:0磷脂酰胆碱等在颈动脉粥样硬化斑块病例组显著高于无斑块对照组。对照组相关分析发现,C24:0鞘磷脂与低密度脂蛋白胆固醇呈显著正相关(r=0.636,P < 0.05),C18:2 (Cis) 磷脂酰胆碱与收缩压呈显著正相关(r=0.733,P < 0.05),C18:0磷脂酰乙醇胺与高敏C反应蛋白呈显著正相关(r=0.782,P < 0.01),C22:0、C24:0神经酰胺及C18:0磷脂酰乙醇胺与蔬菜摄入量呈显著负相关(r=-0.679,P < 0.05;r=-0.711,P < 0.05;r=-0.808,P < 0.01),C24:0神经酰胺与豆类食品摄入量呈显著负相关(r=-0.736,P < 0.05)。结论: 血浆C24:0鞘磷脂、C22:0和C24:0神经酰胺、C18:0磷脂酰乙醇胺、C18:2和C18:0磷脂酰胆碱等脂质代谢分子升高可能是人类动脉粥样硬化斑块的新危险因素,这些分子可能与血脂、血压或炎症水平及蔬菜、豆制品摄入有关,但关联的性质需要在更大样本人群中验证。
中图分类号:
- R543.4
| 1 |
Alizargar J , Bai CH . Factors associated with carotid Intima media thickness and carotid plaque score in community-dwelling and non-diabetic individuals[J]. BMC Cardiovasc Disord, 2018, 18 (1): 21.
doi: 10.1186/s12872-018-0752-1 |
| 2 |
Gonçalves I , Edsfeldt A , Colhoun HM , et al. Association between renin and atherosclerotic burden in subjects with and without type 2 diabetes[J]. BMC Cardiovasc Disord, 2016, 16 (1): 171.
doi: 10.1186/s12872-016-0346-8 |
| 3 |
Lou Y , Li B , Su L , et al. Association between body mass index and presence of carotid plaque among low-income adults aged 45 years and older: A population-based cross-sectional study in rural China[J]. Oncotarget, 2017, 8 (46): 81261- 81272.
doi: 10.18632/oncotarget.17608 |
| 4 | Yang D , Iyer S , Gardener H , et al. Cigarette smoking and carotid plaque echodensity in the Northern Manhattan study[J]. Cerebrovasc Dis, 2015, 40 (3/4): 136- 143. |
| 5 |
Arnold N , Koenig W . Atherosclerosis as an inflammatory disease-pathophysiology, clinical relevance and therapeutic implications[J]. Dtsch Med Wochenschr, 2019, 144 (5): 315- 321.
doi: 10.1055/a-0657-1595 |
| 6 |
谢高强, 于晖, 陈敬洲, 等. 基因变异及心血管危险因素与单核细胞分泌白细胞介素6和10的关系[J]. 北京大学学报(医学版), 2014, 46 (4): 589- 595.
doi: 10.3969/j.issn.1671-167X.2014.04.022 |
| 7 |
Xie G , Myint PK , Zaman MJ , et al. Relationship of serum interleukin-10 and its genetic variations with ischemic stroke in a Chinese general population[J]. PLoS One, 2013, 8 (9): e74126.
doi: 10.1371/journal.pone.0074126 |
| 8 |
Xie G , Myint PK , Zhao L , et al. Relationship between-592A/C polymorphism of interleukin-10 (IL-10) gene and risk of early carotid atherosclerosis[J]. Int J Cardiol, 2010, 143 (1): 102- 104.
doi: 10.1016/j.ijcard.2008.11.173 |
| 9 |
Paapstel K , Kals J , Eha J , et al. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis[J]. Nutr Metab Cardiovasc Dis, 2018, 28 (1): 44- 52.
doi: 10.1016/j.numecd.2017.07.011 |
| 10 |
Pechlaner R , Kiechl S , Mayr M . Potential and caveats of lipidomics for cardiovascular disease[J]. Circulation, 2016, 134 (21): 1651- 1654.
doi: 10.1161/CIRCULATIONAHA.116.025092 |
| 11 |
罗杰斯, 谢高强, 于洋, 等. 中老年人群颈动脉内中膜厚度分布特征及相关因素分析[J]. 中国循环杂志, 2013, 28 (4): 278- 281.
doi: 10.3969/j.issn.1000-3614.2013.04.012 |
| 12 |
Pavoine C , Pecker F . Sphingomyelinases: Their regulation and roles in cardiovascular pathophysiology[J]. Cardiovasc Res, 2009, 82 (2): 175- 183.
doi: 10.1093/cvr/cvp030 |
| 13 |
Kasumov T , Li L , Li M , et al. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis[J]. PLoS One, 2015, 10 (5): e0126910.
doi: 10.1371/journal.pone.0126910 |
| 14 |
Colombaioni L , Garcia-Gil M . Sphingolipid metabolites in neural signalling and function[J]. Brain Res Brain Res Rev, 2004, 46 (3): 328- 355.
doi: 10.1016/j.brainresrev.2004.07.014 |
| 15 |
Kolak M , Westerbacka J , Velagapudi VR , et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity[J]. Diabetes, 2007, 56 (8): 1960- 1968.
doi: 10.2337/db07-0111 |
| 16 |
Zhao W , Wang X , Deik AA , et al. Elevated plasma ceramides are associated with antiretroviral therapy use and progression of carotid artery atherosclerosis in HIV infection[J]. Circulation, 2019, 139 (17): 2003- 2011.
doi: 10.1161/CIRCULATIONAHA.118.037487 |
| 17 | Yin W , Li F , Tan X , et al. Plasma ceramides and cardiovascular events in hypertensive patients at high cardiovascular risk[J]. Am J hypertens, 2020, 34 (11): 1209- 1216. |
| 18 | Jiang XC , Liu J . Sphingolipid metabolism and atherosclerosis[J]. Handb Exp Pharmacol, 2013, (216): 133- 146. |
| 19 | van der Veen JN , Kennelly JP , Wan S , et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease[J]. Biochim Biophys Acta Biomembr, 2017, 1859 (9 Pt B): 1558- 1572. |
| 20 | Vettraino C , Peracchi A , Donini S , et al. Structural characterization of human O-phosphoethanolamine phospho-lyase[J]. Acta Crystallogr F Struct Biol Commun, 2020, 76 (Pt 4): 160- 167. |
| 21 |
Singh SK , Suresh MV , Prayther DC , et al. C-reactive protein-bound enzymatically modified low-density lipoprotein does not transform macrophages into foam cells[J]. J Immunol, 2008, 180 (6): 4316- 4322.
doi: 10.4049/jimmunol.180.6.4316 |
| 22 |
Floegel A , Kühn T , Sookthai D , et al. Serum metabolites and risk of myocardial infarction and ischemic stroke: A targeted metabolomic approach in two German prospective cohorts[J]. Eur J Epidemiol, 2018, 33 (1): 55- 66.
doi: 10.1007/s10654-017-0333-0 |
| 23 |
Park J , Jung TW , Chung YH , et al. 1, 2-Dilinoleoyl-Sn-glycero-3-phosphocholine increases insulin sensitivity in palmitate-treated myotubes and induces lipolysis in adipocytes[J]. Biochem Biophys Res Commun, 2020, 533 (1): 162- 167.
doi: 10.1016/j.bbrc.2020.09.019 |
| 24 |
Yang L , Wang L , Deng Y , et al. Serum lipids profiling perturbances in patients with ischemic heart disease and ischemic cardiomyopathy[J]. Lipids Health Dis, 2020, 19 (1): 89.
doi: 10.1186/s12944-020-01269-9 |
| 25 | Hilvo M , Simolin H , Metso J , et al. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions[J]. Atherosclerosis, 2018, 269, 159- 165. |
| [1] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [2] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [3] | 陈延,李况蒙,洪锴,张树栋,程建星,郑仲杰,唐文豪,赵连明,张海涛,姜辉,林浩成. 阴茎海绵体注射试验对阴茎血管功能影响的回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 680-686. |
| [4] | 庞博,郭桐君,陈曦,郭华棋,石嘉章,陈娟,王欣梅,李耀妍,单安琪,余恒意,黄婧,汤乃军,王艳,郭新彪,李国星,吴少伟. 天津与上海35岁以上人群氮氧化物个体暴露水平及其影响因素[J]. 北京大学学报(医学版), 2024, 56(4): 700-707. |
| [5] | 蔡珊,张依航,陈子玥,刘云飞,党佳佳,师嫡,李佳欣,黄天彧,马军,宋逸. 北京市中小学生身体活动时间现状及影响因素的路径[J]. 北京大学学报(医学版), 2024, 56(3): 403-410. |
| [6] | 张祖洪,陈天娇,马军. 中小学生青春发动时相与心血管代谢危险因素的相关性[J]. 北京大学学报(医学版), 2024, 56(3): 418-423. |
| [7] | 林郁婷,王华丽,田宇,巩俐彤,常春. 北京市老年人认知功能的影响因素[J]. 北京大学学报(医学版), 2024, 56(3): 456-461. |
| [8] | 朱金荣,赵亚娜,黄巍,赵微微,王悦,王松,苏春燕. 感染新型冠状病毒的血液透析患者的临床特征[J]. 北京大学学报(医学版), 2024, 56(2): 267-272. |
| [9] | 赖展鸿,李嘉辰,贠泽霖,张永刚,张昊,邢晓燕,邵苗,金月波,王乃迪,李依敏,李玉慧,栗占国. 特发性炎性肌病完全临床应答相关因素的单中心真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 284-292. |
| [10] | 司筱芊,赵秀娟,朱凤雪,王天兵. 创伤出血性休克后急性呼吸窘迫综合征的危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 307-312. |
| [11] | 李洋洋,侯林,马紫君,黄山雅美,刘捷,曾超美,秦炯. 孕期因素与婴儿牛奶蛋白过敏的关系[J]. 北京大学学报(医学版), 2024, 56(1): 144-149. |
| [12] | 刘晓强,周寅. 牙种植同期植骨术围术期高血压的相关危险因素[J]. 北京大学学报(医学版), 2024, 56(1): 93-98. |
| [13] | 罗靓,李云,王红彦,相晓红,赵静,孙峰,张晓盈,贾汝琳,李春. 抗内皮细胞抗体检测在早期流产中的预测价值[J]. 北京大学学报(医学版), 2023, 55(6): 1039-1044. |
| [14] | 游芳凝,罗靓,刘香君,张学武,李春. 未分化结缔组织病患者的妊娠结局、疾病演变及其影响因素[J]. 北京大学学报(医学版), 2023, 55(6): 1045-1052. |
| [15] | 李宇菲,闫亚妮,靳家扬,李春,裴秋艳. 合并胎儿心脏病变的抗SSA抗体阳性孕妇的临床及实验室特征[J]. 北京大学学报(医学版), 2023, 55(6): 1053-1057. |
|
||