北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (5): 919-925. doi: 10.19723/j.issn.1671-167X.2025.05.016
泛素特异性蛋白酶35对类风湿关节炎成纤维样滑膜细胞铁死亡的作用及机制
- 厦门市第五医院风湿免疫科,福建厦门 361101
Role and mechanism of ubiquitin-specific protease 35 in ferroptosis of rheumatoid arthritis-fibroblast like synoviocytes
Lianghua FENG*( ), Lirong HONG, Yujia CHEN, Xueming CAI
), Lirong HONG, Yujia CHEN, Xueming CAI
- Department of Rheumatology and Immunology, Xiamen Fifth Hospital, Xiamen 361101, Fujian, China
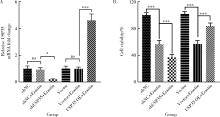
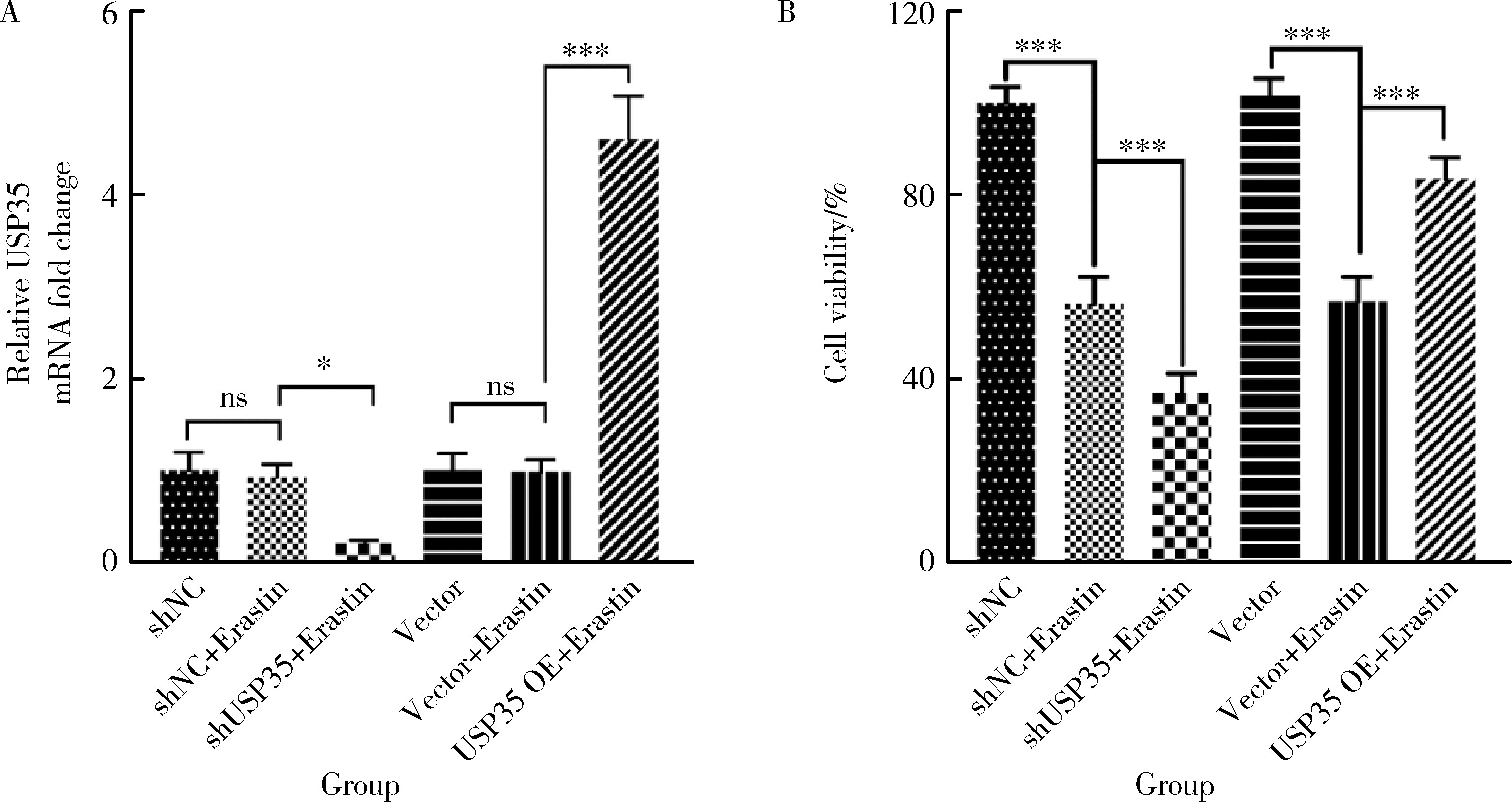
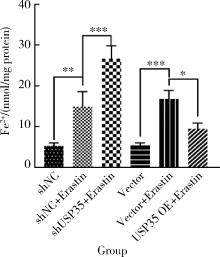
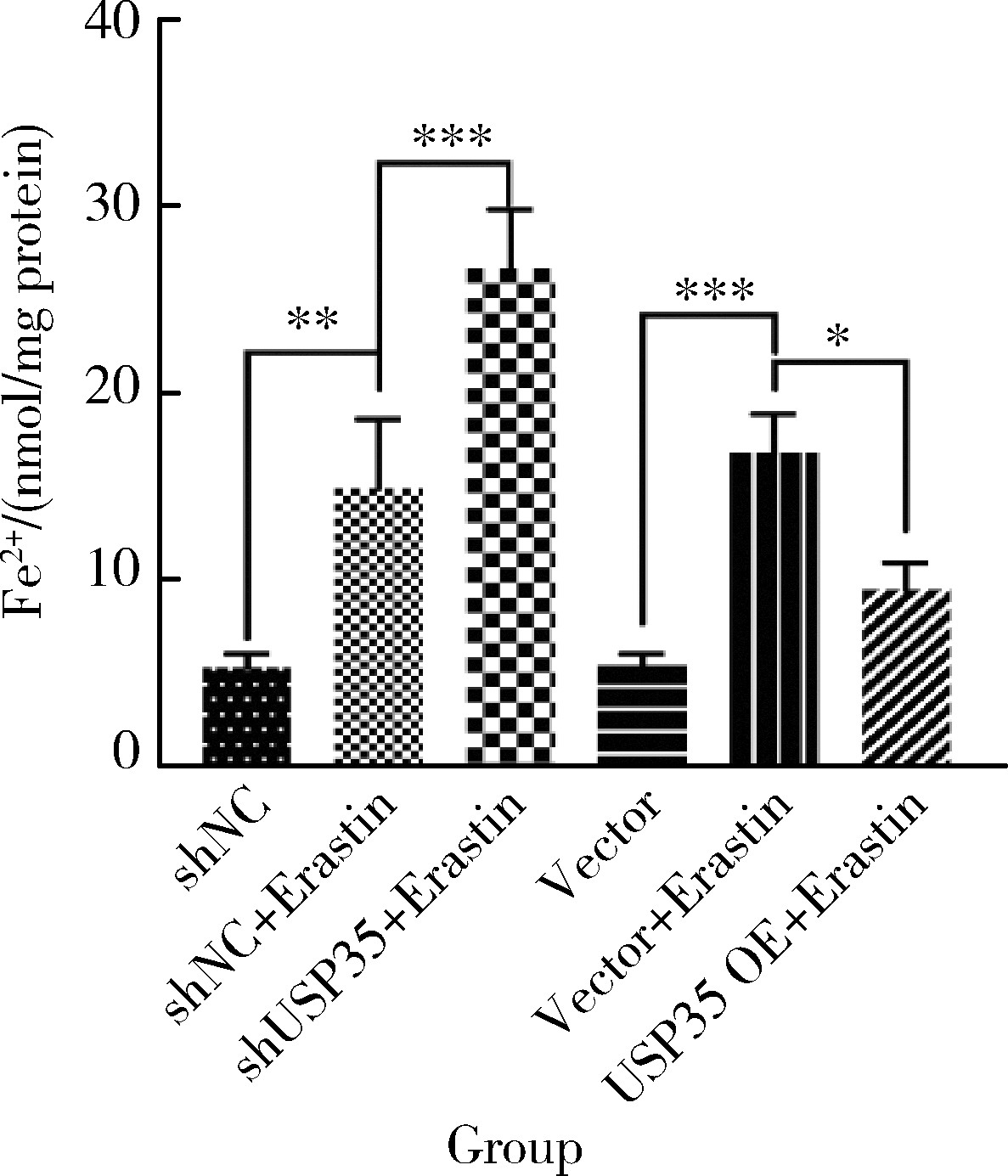
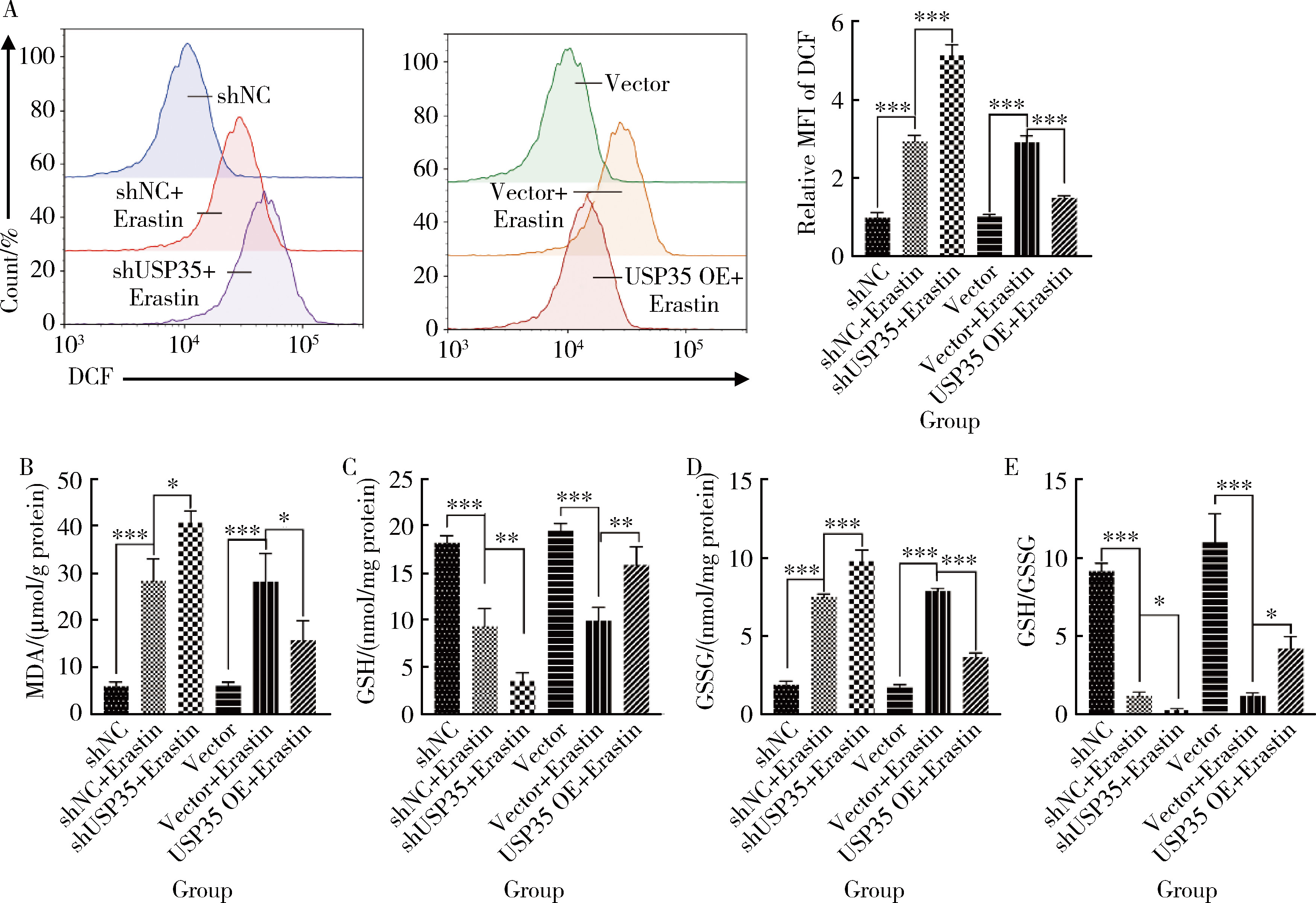
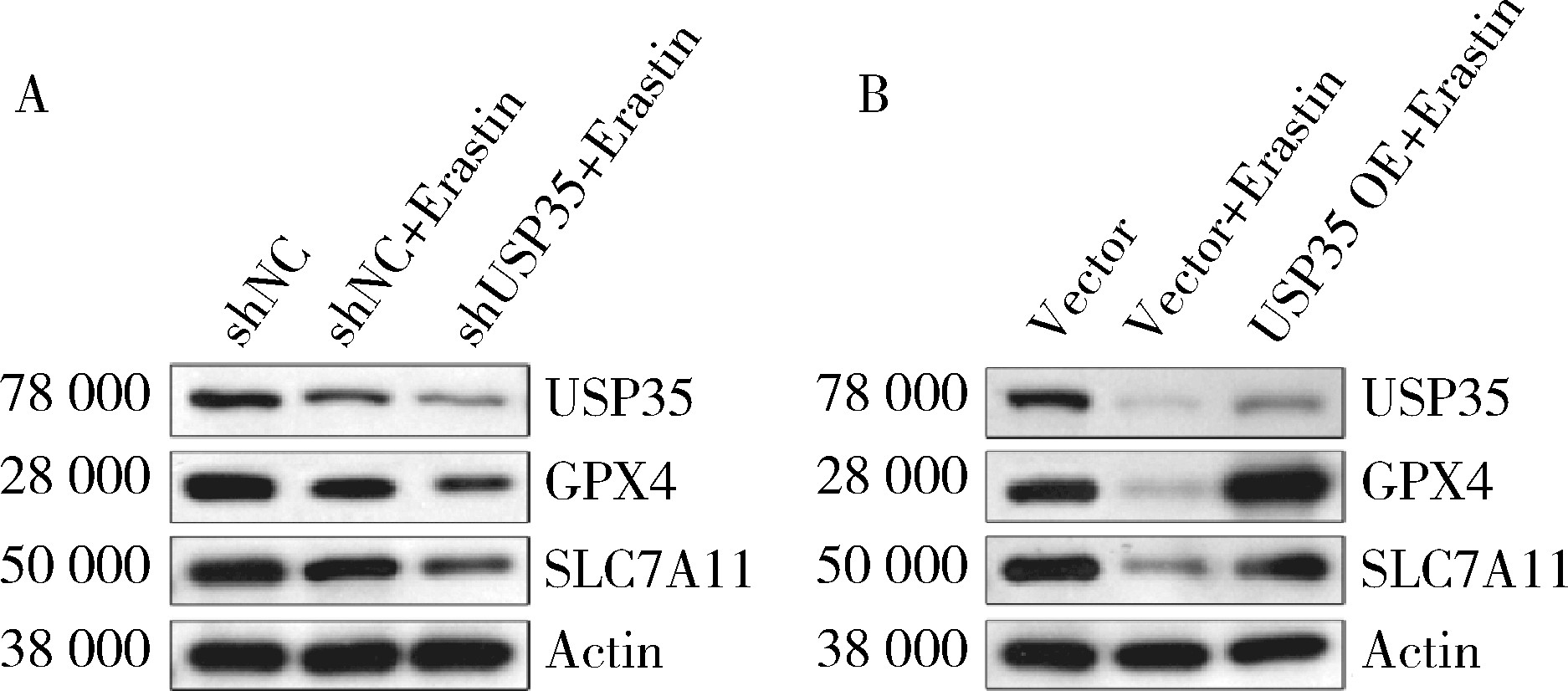
摘要: 目的: 研究泛素特异性蛋白酶35(ubiquitin-specific protease 35, USP35)对类风湿关节炎成纤维样滑膜细胞(rheumatoid arthritis-fibroblast like synoviocytes, RA-FLS)铁死亡的作用及机制,加深对RA发病机制的理解,并为其治疗提供潜在的标靶。方法: (1) 体外培养RA-FLS,用慢病毒载体感染以构建稳定敲低USP35的细胞系(short hairpin ribonucleic acid of USP35,shUSP35)和其对照细胞系(negtive control of short hairpin ribonucleic acid,shNC),以及稳定过表达USP35的细胞系(overexpression of USP35,USP35 OE)和其对照细胞系(Vector)。为了探究USP35在铁死亡调控中的作用,使用1 μmol/L依拉司亭(Erastin)诱导RA-FLS细胞构建铁死亡模型。将细胞分为6组,第一组是shNC细胞,第二组是用Erastin处理shNC细胞(shNC+Erastin),第三组是用Erastin处理shUSP35细胞(shUSP35+Erastin),第四组是Vector细胞,第五组是用Erastin处理Vector细胞(Vector+Erastin),第六组是用Erastin处理USP35 OE细胞(USP35 OE+Erastin)。(2)采用细胞计数试剂盒-8(cell counting kit-8, CCK8)检测细胞活力;(3)分别使用活性氧(reactive oxygen species, ROS)检测试剂盒、丙二醛(malondialdehyde, MDA)检测试剂盒、谷胱甘肽(glutathione, GSH)和氧化型谷胱甘肽(glutathione sulfide,GSSG)检测试剂盒、亚铁离子含量检测试剂盒检测细胞中ROS、MDA、Fe2+的含量以及GSH/GSSG比值;(4)采用蛋白免疫印迹实验(Western blotting)检测溶质载体家族7成员11(solute carrier family 7 member 11,SLC7A11)和谷胱甘肽过氧化物酶4(glutathione peroxidase 4, GPX4)蛋白的表达水平。结果: (1) 与shNC+Erastin组相比,shUSP35+Erastin组的RA-FLS细胞活力显著降低(P<0.001),而与Vector+Erastin组相比,USP35 OE+Erastin组的细胞活力显著升高(P<0.001),表明USP35可显著缓解Erastin对RA-FLS细胞活力的抑制作用。(2)与shNC+Erastin组相比,shUSP35+Erastin组细胞的ROS(P<0.001)、MDA(P<0.05)和Fe2+水平(P<0.001)显著升高,GSH/GSSG比值显著增加(P<0.05);而与Vector+Erastin组相比,USP35OE+Erastin组细胞的ROS(P<0.001)、MDA(P<0.05)和Fe2+水平(P<0.05)显著降低,GSH/GSSG比值显著下降(P<0.05),表明USP35可显著抑制Erastin诱导的RA-FLS细胞氧化应激和脂质过氧化。(3)在Erastin诱导的RA-FLS中,USP35的表达与SLC7A11和GPX4蛋白水平呈正相关。结论: USP35抑制RA-FLS的铁死亡,可能与增加SLC7A11和GPX4表达相关。
中图分类号:
- R593.22
| 1 |
doi: 10.1038/s41419-020-2314-6 |
| 2 |
doi: 10.1186/ar2669 |
| 3 |
doi: 10.1038/nature01661 |
| 4 |
doi: 10.1111/j.0105-2896.2009.00859.x |
| 5 |
doi: 10.1016/j.coph.2022.102304 |
| 6 |
doi: 10.1038/nrrheum.2012.190 |
| 7 |
doi: 10.1038/s41584-020-0413-5 |
| 8 |
doi: 10.1016/j.cell.2012.03.042 |
| 9 |
doi: 10.3389/fimmu.2023.1260839 |
| 10 |
doi: 10.1038/s41467-021-27948-4 |
| 11 |
doi: 10.1080/15548627.2020.1810918 |
| 12 |
doi: 10.1016/j.tcb.2015.10.014 |
| 13 |
doi: 10.1038/s41418-023-01176-3 |
| 14 |
doi: 10.1002/ctm2.390 |
| 15 |
doi: 10.1016/j.lfs.2020.118459 |
| 16 |
doi: 10.1186/s13046-023-02805-y |
| 17 |
doi: 10.1038/s41418-021-00907-8 |
| 18 |
doi: 10.1016/j.freeradbiomed.2020.10.307 |
| 19 |
doi: 10.3389/fimmu.2022.955069 |
| 20 |
doi: 10.1038/cdd.2015.158 |
| 21 |
doi: 10.3389/fimmu.2022.779585 |
| 22 |
doi: 10.3389/fimmu.2023.1197275 |
| [1] | 杨菊, 徐婧, 戴菊华, 石连杰. Lumican蛋白在类风湿关节炎患者血清中的表达及其与疾病和免疫活动的相关性[J]. 北京大学学报(医学版), 2025, 57(5): 911-918. |
| [2] | 许秋实, 刘彤, 王俊杰. 铁死亡相关长链非编码核糖核酸预测放射治疗后非小细胞肺癌患者的临床结局[J]. 北京大学学报(医学版), 2025, 57(3): 569-577. |
| [3] | 贾霈雯, 杨迎, 邹耀威, 欧阳志明, 林建子, 马剑达, 杨葵敏, 戴冽. 类风湿关节炎患者低肌肉量综合征的临床特征及其对躯体功能的影响[J]. 北京大学学报(医学版), 2024, 56(6): 1009-1016. |
| [4] | 马豆豆, 卢哲敏, 郭倩, 朱莎, 古今, 丁艳, 石连杰. 小剂量利妥昔单抗成功治疗类风湿关节炎合并重症肌无力1例[J]. 北京大学学报(医学版), 2024, 56(6): 1110-1114. |
| [5] | 赵柯林, 夏雪, 史乃旭, 周韩, 盖婧雯, 李萍. 铁死亡标志物4-HNE在系统性硬化症细胞模型中的表达及意义[J]. 北京大学学报(医学版), 2024, 56(6): 950-955. |
| [6] | 闫蕊, 柯丹, 张妍, 李丽, 苏焕然, 陈伟, 孙明霞, 刘晓敏, 罗靓. 血清趋化因子CXCL-10和涎液化糖链抗原6水平在类风湿关节炎合并肺间质病变患者中的诊断和病情评估价值[J]. 北京大学学报(医学版), 2024, 56(6): 956-962. |
| [7] | 赵亮, 史成龙, 马柯, 赵静, 王潇, 邢晓燕, 莫万星, 练益瑞, 高超, 李玉慧. 抗合成酶综合征重叠类风湿关节炎患者的免疫学特征[J]. 北京大学学报(医学版), 2024, 56(6): 972-979. |
| [8] | 韩艺钧, 陈小莉, 李常虹, 赵金霞. 甲氨蝶呤在类风湿关节炎患者中的应用现状[J]. 北京大学学报(医学版), 2024, 56(6): 994-1000. |
| [9] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [10] | 黄会娜,赵静,赵祥格,白自然,李霞,王冠. 乳酸对类风湿关节炎患者外周血CD4+T细胞亚群的调控作用[J]. 北京大学学报(医学版), 2024, 56(3): 519-525. |
| [11] | 汤晓菲,李永红,丁秋玲,孙卓,张阳,王育梅,田美伊,刘坚. 类风湿关节炎患者下肢深静脉血栓发病率及危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 279-283. |
| [12] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [13] | 吴琦,蔡月明,何娟,黄文蒂,王庆文. 血脂异常与类风湿关节炎肺间质病变的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 982-992. |
| [14] | 张警丰,金银姬,魏慧,姚中强,赵金霞. 体重指数与类风湿关节炎临床特征的相关性分析[J]. 北京大学学报(医学版), 2023, 55(6): 993-999. |
| [15] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
|
||