北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (6): 1051-1060. doi: 10.19723/j.issn.1671-167X.2025.06.006
利妥昔单抗治疗原发性干燥综合征肾损害的临床疗效和安全性
赵亚云1,2,*, 倪梦凡3,*, 李雪1, 王蓓1,4, 程功1, 何菁1, 金月波1,*( )
)
- 1. 北京大学人民医院风湿免疫科,北京 100044
2. 河北省中医院风湿病科,石家庄 050000
3. 北京大学人民医院肾内科,北京 100044
4. 贵州省黔东南州人民医院风湿免疫科,贵州凯里 556000
Clinical efficacy and safety of rituximab in treating renal injury in primary Sjögren syndrome
Yayun ZHAO1,2, Mengfan NI3, Xue LI1, Bei WANG1,4, Gong CHENG1, Jing HE1, Yuebo JIN1,*( )
)
- 1. Department of Rheumatology and Immunology, Peking University People' s Hospital, Beijing 100044, China
2. Department of Rheumatology, Hebei Provincial Hospital of Chinese Medicine, Shijiazhuang 050000, China
3. Department of Nephrology, Peking University People' s Hospital, Beijing 100044, China
4. Department of Rheumatology and Immunology, Qiandongnan People' s Hospital, Kaili 556000, Guizhou, China
摘要:
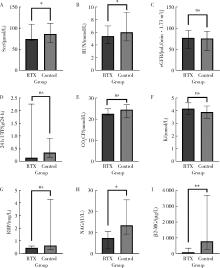
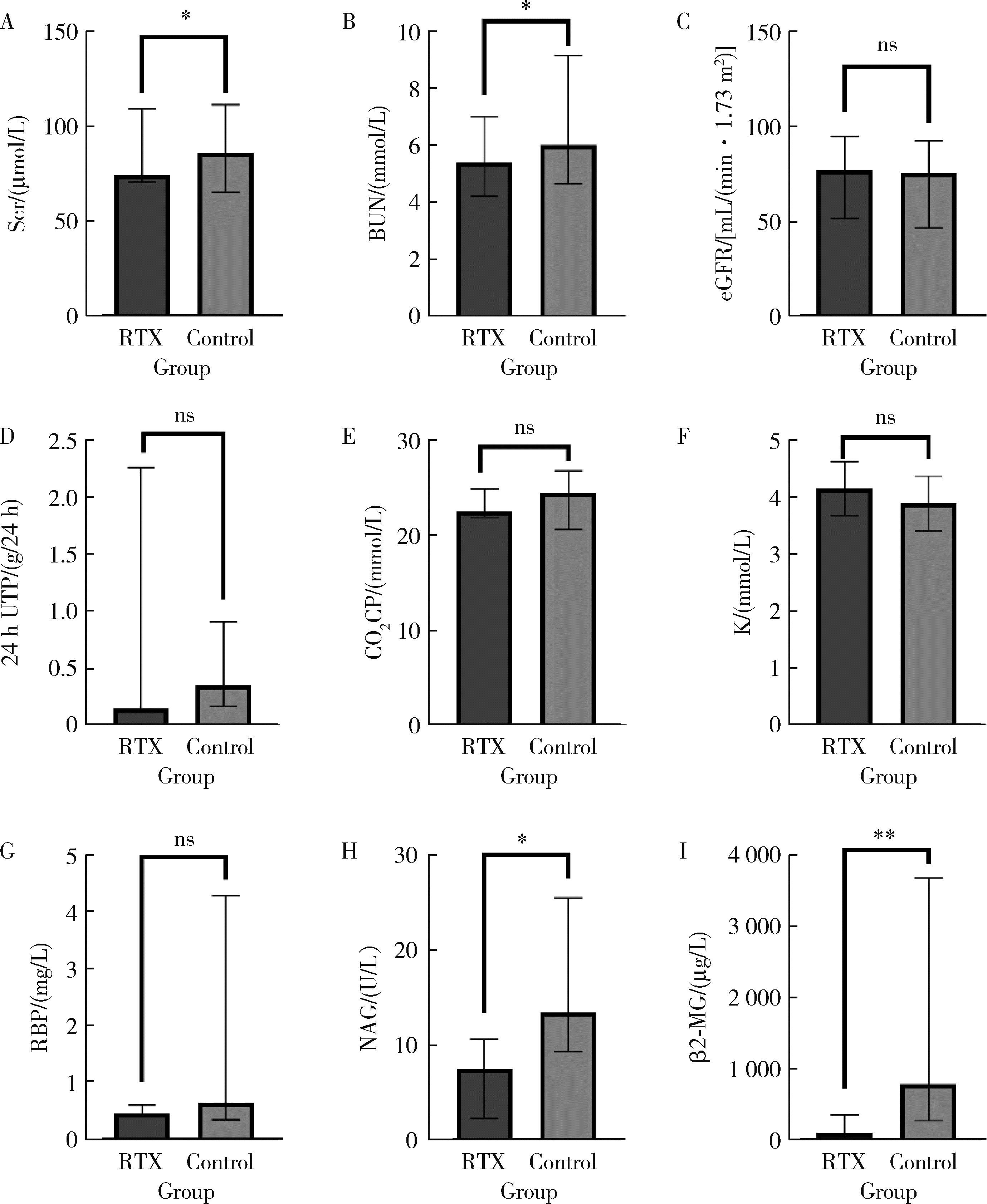
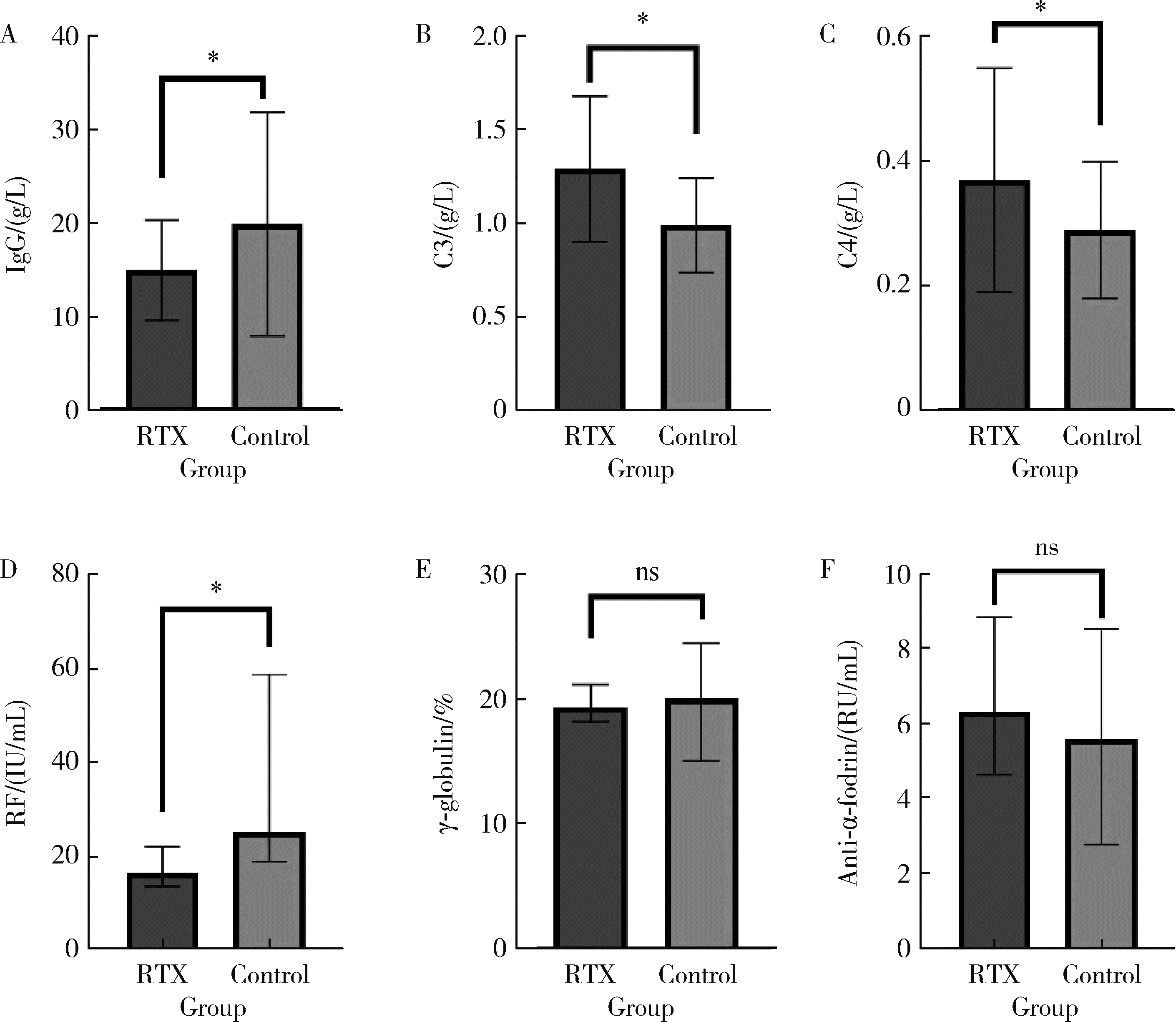
目的: 肾损害是原发性干燥综合征(primary Sjögren syndrome,pSS)常见的腺体外损伤之一,总体预后不良,但早期免疫治疗有助于减轻肾损伤,改善远期肾功能。越来越多的证据表明,利妥昔单抗(rituximab,RTX)治疗干燥综合征的系统损害有效。本研究通过回顾性分析,探索RTX治疗pSS肾损害的临床疗效和安全性。方法: 检索北京大学人民医院临床大数据应用平台,连续纳入2013年7月至2025年1月在北京大学人民医院风湿免疫科和肾内科就诊,且应用RTX治疗的pSS继发肾损害的患者17例,匹配同期年龄、性别、基线病情相当的,应用传统免疫抑制药物治疗的患者34例,收集所有患者的临床及实验室数据,RTX组患者接受糖皮质激素联合RTX治疗,对照组患者接受糖皮质激素联合传统免疫抑制药物进行治疗,分析治疗6个月后的病情变化,对两组患者治疗前后的一般实验室检查结果、肾损害指标、免疫学指标等进行比较。结果: 治疗6个月后,肾损害指标方面,RTX组患者尿N-乙酰β-D氨基葡萄糖苷酶、尿β2微球蛋白、肌酐、尿素氮均显著低于对照组( P < 0.01、P < 0.05),24小时尿蛋白水平、尿视黄醇结合蛋白水平低于对照组,血钾高于对照组;免疫学指标方面,RTX组免疫球蛋白G (immunoglobulin G, IgG)、类风湿因子(rheumatoid factor, RF)水平明显低于对照组( P < 0.05),补体C3、C4明显高于对照组( P < 0.05);RTX组的欧洲抗风湿病联盟干燥综合征疾病活动指数(European League Against Rheumatism Sjögren syndrome disease activity index,ESSDAI)总分和肾脏评分均明显低于对照组,差异有统计学意义( P < 0.05)。分析两组患者治疗6个月时的激素减量情况,RTX组的泼尼松减至小剂量(0~5 mg,每日1次)的患者比例高于对照组(64.71% vs. 32.35%,P=0.038)。比较两组患者半年内的感染发生率,RTX组为1/17例,对照组为3/34例,两组均未见严重的不良反应。结论: RTX可通过靶向清除致病性B细胞,有效治疗pSS患者的肾小管间质以及肾小球损害,改善肾脏代谢及酸碱平衡功能,其治疗效果可能优于传统免疫抑制药物,有助于激素减量,且患者的依从性、安全性良好,具有较好的临床应用前景。
中图分类号:
- R593.2
| 1 |
doi: 10.1056/NEJMcp1702514 |
| 2 |
doi: 10.2215/CJN.00980209 |
| 3 |
doi: 10.1002/art.38100 |
| 4 |
doi: 10.1136/annrheumdis-2019-216114 |
| 5 |
doi: 10.1186/ar4359 |
| 6 |
doi: 10.1038/s41584-024-01135-3 |
| 7 |
|
| 8 |
|
| 9 |
北京大学医学部肾脏病学系专家组. 利妥昔单抗在膜性肾病中应用的专家共识[J]. 中华内科杂志, 2022, 61(3): 282- 290.
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
doi: 10.1038/s41573-020-00092-2 |
| 14 |
doi: 10.1172/JCI40231 |
| 15 |
doi: 10.1002/art.22536 |
| 16 |
doi: 10.1136/annrheumdis-2011-200086 |
| 17 |
doi: 10.7326/M13-1085 |
| 18 |
doi: 10.1007/s10067-005-0086-0 |
| 19 |
doi: 10.1177/0961203314546023 |
| 20 |
中国初级卫生保健基金会风湿免疫学专业委员会. 干燥综合征超药品说明书用药中国临床实践指南(2023版)[J]. 中华医学杂志, 2023, 103(43): 3445- 3461.
|
| 21 |
中国初级卫生保健基金会风湿免疫学分会干燥综合征和IgG4相关性疾病专委会, 系统性红斑狼疮专委会. 利妥昔单抗治疗风湿免疫病中国专家共识(2024版)[J]. 中华风湿病学杂志, 2024, 28(8): 521- 537.
|
| 22 |
doi: 10.3389/fphar.2021.731122 |
| [1] | 刘源, 石桂秀. 干燥综合征到干燥病的命名变迁[J]. 北京大学学报(医学版), 2025, 57(6): 1015-1017. |
| [2] | 林文灏, 谢阳, 王芳晴, 王淑盈, 刘香君, 胡凡磊, 贾园. 基于B细胞单细胞转录组测序的干燥综合征分子分型[J]. 北京大学学报(医学版), 2025, 57(6): 1032-1041. |
| [3] | 向钊, 杨莉, 杨静. 非靶向代谢组学揭示原发性干燥综合征血小板减少患者血清差异代谢物及代谢通路[J]. 北京大学学报(医学版), 2025, 57(6): 1042-1050. |
| [4] | 丁艳, 王丽芳, 李超然, 卢哲敏, 石连杰. 利妥昔单抗成功治疗类风湿关节炎合并IgG4相关性疾病1例[J]. 北京大学学报(医学版), 2025, 57(6): 1203-1207. |
| [5] | 朱丽秀, 陈仁利, 周素娟, 林烨, 汤一榕, 叶桢. 水通道蛋白5对干燥综合征大鼠TLR4/MyD88/NF-κB信号的影响[J]. 北京大学学报(医学版), 2025, 57(5): 875-883. |
| [6] | 宁圆, 张晓盈, 李雪, 李原, 何菁, 金月波. 干燥综合征并发乳腺淋巴瘤1例[J]. 北京大学学报(医学版), 2025, 57(4): 808-811. |
| [7] | 王紫薇, 李闵, 高慧, 邓芳. 链球菌感染与过敏性紫癜肾炎患儿肾损害的相关性[J]. 北京大学学报(医学版), 2025, 57(2): 284-290. |
| [8] | 马豆豆, 卢哲敏, 郭倩, 朱莎, 古今, 丁艳, 石连杰. 小剂量利妥昔单抗成功治疗类风湿关节炎合并重症肌无力1例[J]. 北京大学学报(医学版), 2024, 56(6): 1110-1114. |
| [9] | 杨玉淑, 齐晅, 丁萌, 王炜, 郭惠芳, 高丽霞. 抗唾液腺蛋白1抗体联合抗腮腺分泌蛋白抗体对干燥综合征的诊断价值[J]. 北京大学学报(医学版), 2024, 56(5): 845-852. |
| [10] | 韩艺钧,李常虹,陈秀英,赵金霞. 抗SSB抗体阳性和阴性的原发性干燥综合征患者临床及免疫学特征的比较[J]. 北京大学学报(医学版), 2023, 55(6): 1000-1006. |
| [11] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [12] | 孟彦宏,陈怡帆,周培茹. CENP-B抗体阳性的原发性干燥综合征患者的临床和免疫学特征[J]. 北京大学学报(医学版), 2023, 55(6): 1088-1096. |
| [13] | 吴洁,张雯,梁舒,秦艺璐,范文强. 妊娠期原发性干燥综合征合并视神经脊髓炎谱系疾病危重症1例[J]. 北京大学学报(医学版), 2023, 55(6): 1118-1124. |
| [14] | 王丽芳,石连杰,宁武,高乃姝,王宽婷. 干燥综合征合并冷凝集素病1例[J]. 北京大学学报(医学版), 2023, 55(6): 1130-1134. |
| [15] | 邢海霞,王琳,乔迪,刘畅,潘洁. 干燥综合征口腔疾病的治疗特点[J]. 北京大学学报(医学版), 2023, 55(5): 929-933. |
|
||