北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (2): 234-238. doi: 10.19723/j.issn.1671-167X.2019.02.006
Rasfonin抑制骨肉瘤细胞143B的增殖和迁移
- 1. 北京大学人民医院骨肿瘤科, 北京 100044
2. 郑州大学附属肿瘤医院骨软组织科, 郑州 450008
Rasfonin inhibits proliferation and migration of osteosarcoma 143B cells
Fan ZHANG1,2,Tai-qiang YAN1,∆( ),Wei GUO1
),Wei GUO1
- 1. Musculoskeletal Tumor Center, Peking University People’s Hospital, Beijing 100044, China;
2. Department of Bone and Soft Tissue, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, China
摘要:
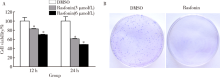
目的: 研究真菌次级代谢产物rasfonin对骨肉瘤143B细胞增殖与迁移能力的影响。方法: 以骨肉瘤细胞系143B为模型,利用3-(4,5-二甲基) -5-(3-羧甲基苯环)-2-(4-硫基苯)-2H-四唑盐复合物检测法[3-(4,5-dime-thylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, MTS]观察rasfonin对细胞活性的影响,以二甲基亚砜(dimethyl sulfoxide,DMSO)组为对照,采用rasfonin (3 μmol/L和6 μmol/L)处理12或24 h,观察143B细胞活性的变化;采用克隆形成实验检测rasfonin对细胞集落形成能力的影响,以DMSO组为对照,采用rasfonin (3 μmol/L)处理1周,对比两组克隆形成数目。划痕实验及侵袭实验检测rasfonin对细胞侵袭迁移能力的影响,以DMSO组为对照,采用rasfonin (3 μmol/L)处理24 h,对比两组的伤痕愈合率及侵袭细胞数。以DMSO组为对照,透射电子显微镜观察rasfonin(3 μmol/L)处理4 h后细胞内自噬体含量的变化。蛋白免疫印迹的方法检测rasfonin对p62蛋白(sequestosome 1)、微管相关蛋白1轻链3融合蛋白(microtubule associated protein 1 light chain 3 fusion protein, LC3)及聚腺苷酸二磷酸核糖转移酶[poly (ADP-ribose) polymerase-1, PARP-1]表达的影响。结果: 在12和24 h,随着rasfonin浓度的升高,骨肉瘤143B细胞的细胞活性均逐渐降低,3组间差异有统计学意义(12 h: F=31.36, P<0.01; 24 h: F=67.07, P<0.01),任意两组间比较差异也均有统计学意义(P<0.01)。3 μmol/L的rasfonin可以显著抑制143B细胞的集落形成能力(P<0.01)。Rasfonin处理后,143B细胞在24 h内的伤痕愈合比率明显低于DMSO对照组(33.91%±0.83% vs. 65.11%±0.94%, P<0.01);同样24 h内穿过覆盖有Matrigel的基底膜的细胞数明显低于DMSO对照组[(21.33±1.45)个vs.(49.33±2.40)个, P<0.01]。Rasfonin处理4 h后骨肉瘤143B细胞中自噬体增多,p62水平降低,LC3-Ⅱ累积,自噬抑制剂氯喹存在条件下LC3-Ⅱ累积更为明显,并且随着rasfonin浓度的提高PARP-1的切割增多。结论: Rasfonin既可以抑制骨肉瘤143B细胞的增殖迁移,还可以引起细胞的自噬与凋亡,这些结果为rasfonin作为骨肉瘤新的治疗药物提供了初步的实验基础。
中图分类号:
- R738.1
| [1] |
Osasan S, Zhang M, Shen F , et al. Osteogenic sarcoma: a 21st century review[J]. Anticancer Res, 2016,36(9):4391-4398.
doi: 10.21873/anticanres |
| [2] |
Shaikh AB, Li F, Li M , et al. Present advances and future perspectives of molecular targeted therapy for osteosarcoma[J]. Int J Mol Sci, 2016,17(4):506.
doi: 10.3390/ijms17040506 |
| [3] |
Tomikawa T, Shin-Ya K, Furihata K , et al. Rasfonin, a new apoptosis inducer in ras-dependent cells from Talaromyces sp.[J]. J Antibiot (Tokyo), 2000,53(8):848-850.
doi: 10.7164/antibiotics.53.848 |
| [4] |
Xiao Z, Li L, Li Y , et al. Rasfonin, a novel 2-pyrone derivative, induces ras-mutated Panc-1 pancreatic tumor cell death in nude mice[J]. Cell Death Dis, 2014,5(5):e1241.
doi: 10.1038/cddis.2014.213 |
| [5] |
Lu Q, Yan S, Sun H , et al. Akt inhibition attenuates rasfonin-induced autophagy and apoptosis through the glycolytic pathway in renal cancer cells[J]. Cell Death Dis, 2015,6(12):e2005.
doi: 10.1038/cddis.2015.344 |
| [6] |
Ouyang L, Shi Z, Zhao S , et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis[J]. Cell Prolif, 2012,45(6):487-498.
doi: 10.1111/cpr.2012.45.issue-6 |
| [7] |
Hale AN, Ledbetter DJ, Gawriluk TR , et al. Autophagy: regulation and role in development[J]. Autophagy, 2013,9(7):951-972.
doi: 10.4161/auto.24273 |
| [8] |
Levine B, Yuan J . Autophagy in cell death: an innocent convict?[J]. J Clin Invest, 2005,115(10):2679-2688.
doi: 10.1172/JCI26390 |
| [9] |
Eisenberg-Lerner A, Bialik S, Simon HU , et al. Life and death partners: apoptosis, autophagy and the cross-talk between them[J]. Cell Death Differ, 2009,16(7):966-975.
doi: 10.1038/cdd.2009.33 |
| [10] | 燕太强, 梁伟民, 郭卫 . 骨肉瘤的诊疗和研究进展[J]. 中华临床医师杂志: 电子版, 2012(17):4988-4990. |
| [11] |
Wang W, Sun H, Che Y , et al. Rasfonin promotes autophagy and apoptosis via upregulation of reactive oxygen species (ros)/jnk pathway[J]. Mycology, 2016,7(2):64.
doi: 10.1080/21501203.2016.1170073 |
| [12] |
Galluzzi L, Vitale I, Abrams JM , et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012[J]. Cell Death Differ, 2012,19(1):107-120.
doi: 10.1038/cdd.2011.96 |
| [13] |
Kanzawa T, Germano IM, Komata T , et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells[J]. Cell Death Differ, 2004,11(4):448-457.
doi: 10.1038/sj.cdd.4401359 |
| [14] |
Shinojima N, Yokoyama T, Kondo Y , et al. Roles of the akt/mtor/p70s6k and erk1/2 signaling pathways in curcumin-induced autophagy[J]. Autophagy, 2007,3(6):635-637.
doi: 10.4161/auto.4916 |
| [15] |
曹珮, 姜学军, 席志军 . 舒尼替尼通过抑制 Akt/mTOR 信号通路诱导肾癌细胞自噬[J]. 北京大学学报(医学版), 2016,48(4):584-589.
doi: 10.3969/j.issn.1671-167X.2016.04.003 |
| [1] | 张瑶,郭金鑫,战世佳,洪恩宇,杨慧,贾安娜,常艳,郭永丽,张璇. 富含半胱氨酸和甘氨酸蛋白2在神经母细胞瘤恶性进展中的功能和机制[J]. 北京大学学报(医学版), 2024, 56(3): 495-504. |
| [2] | 曹钟,岑红兵,赵建红,梅俊,秦灵芝,廖伟,敖启林. 胰腺神经内分泌肿瘤和实性假乳头状肿瘤中INSM1和SOX11的表达及意义[J]. 北京大学学报(医学版), 2023, 55(4): 575-581. |
| [3] | 王磊,韩天栋,江卫星,李钧,张道新,田野. 主动迁移技术与原位碎石技术在输尿管软镜治疗1~2 cm输尿管上段结石中的安全性和有效性比较[J]. 北京大学学报(医学版), 2023, 55(3): 553-557. |
| [4] | 刘媛,原婉琼,李婷,王平章,吕平,吴利新,阮国瑞,韩文玲,莫晓宁. 敲减CMTM3增加急性B淋巴细胞白血病细胞对伊马替尼敏感性[J]. 北京大学学报(医学版), 2022, 54(6): 1238-1243. |
| [5] | 来丽叶,窦长松,智翠娜,陈洁,马雪,赵鹏,姚碧云. 姜黄素干预通过促进自噬改善氯化锰所致的大鼠神经行为损伤[J]. 北京大学学报(医学版), 2022, 54(3): 400-411. |
| [6] | 杨朵,周心娜,王硕,王小利,袁艳华,杨化兵,耿会珍,彭兵,李子博,李彬,任军. 树突状细胞疫苗特异肿瘤多肽联合树突状细胞体外刺激淋巴细胞功能评估[J]. 北京大学学报(医学版), 2021, 53(6): 1094-1098. |
| [7] | 王子乔,刘燕鹰,张霞,刘田,任立敏,沈丹华,王屹,栗占国. 17例误诊为IgG4相关疾病患者的临床特点及误诊原因分析[J]. 北京大学学报(医学版), 2019, 51(6): 1025-1031. |
| [8] | 姚心韵,高晓敏,邹晓英,岳林. 胞内转运对根尖牙乳头干细胞表面CXC趋化因子受体4表达的影响[J]. 北京大学学报(医学版), 2019, 51(5): 893-899. |
| [9] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [10] | 季兰岚,郝燕捷,张卓莉. 原发性骨髓纤维化引起的继发性痛风1例[J]. 北京大学学报(医学版), 2018, 50(6): 1117-1119. |
| [11] | 孙静,宋卫东,闫思源,席志军. 氯喹抑制肾癌细胞活性促进舒尼替尼诱导的细胞凋亡[J]. 北京大学学报(医学版), 2018, 50(5): 778-784. |
| [12] | 王子成,程立,吕同德,苏黎,林健,周利群. 炎症因子预处理的脂肪干细胞可明显抑制外周血单个核细胞增殖[J]. 北京大学学报(医学版), 2018, 50(4): 590-594. |
| [13] | 唐旭,赵卫红,宋琴琴,殷华奇,杜依青,盛正祚,王强,张晓威,李清,刘士军,徐涛. SOX10对前列腺癌细胞增殖及侵袭的影响[J]. 北京大学学报(医学版), 2018, 50(4): 602-606. |
| [14] | 汪晓彤,饶南荃,方腾姣子,赵玉鸣,葛立宏. 乳牙牙髓干细胞CD146阳性/阴性细胞亚群生物学特性的比较[J]. 北京大学学报(医学版), 2018, 50(2): 284-292. |
| [15] | 陈玮, 胡凡磊, 刘洪江, 徐丽玲, 李英妮, 栗占国. 类风湿关节炎患者髓系来源的抑制细胞促进自身B细胞增殖[J]. 北京大学学报(医学版), 2017, 49(5): 819-823. |
|
||