北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (6): 1108-1114. doi: 10.19723/j.issn.1671-167X.2019.06.023
比较矿物三氧化物凝聚体及山东蜂胶乙醇提取物对牙髓成纤维细胞生物学性能的影响
- 北京大学口腔医学院·口腔医院,儿童口腔科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Effects of mineral trioxide aggregate and ethanolic extracts of Shandong propolis on the biological properties of human dental pulp fibroblasts
Bing-qing SHI,Xiao-jing YUAN,Yu-ming ZHAO( )
)
- Department of Pediatric Dentistry, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
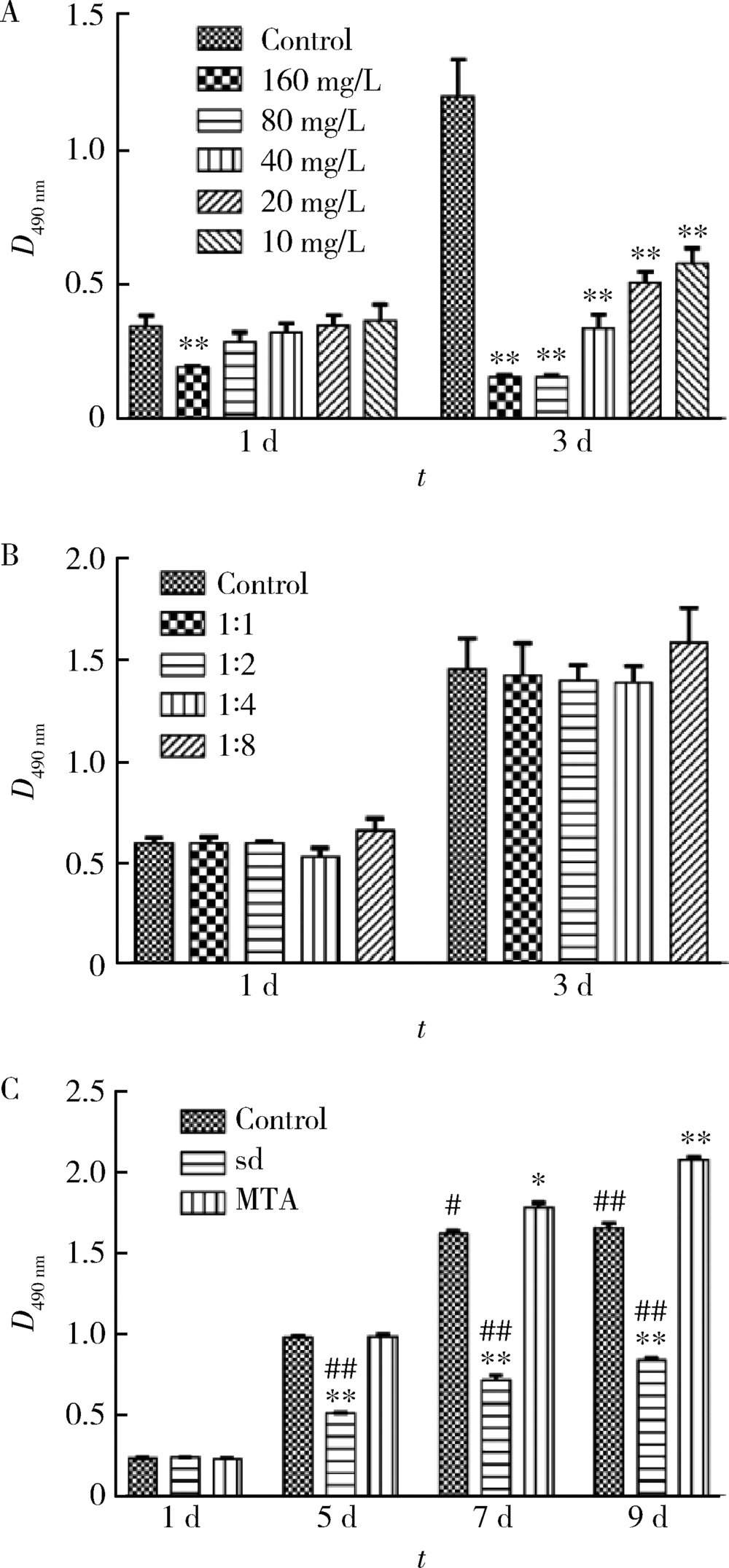
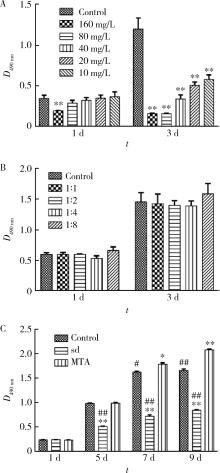
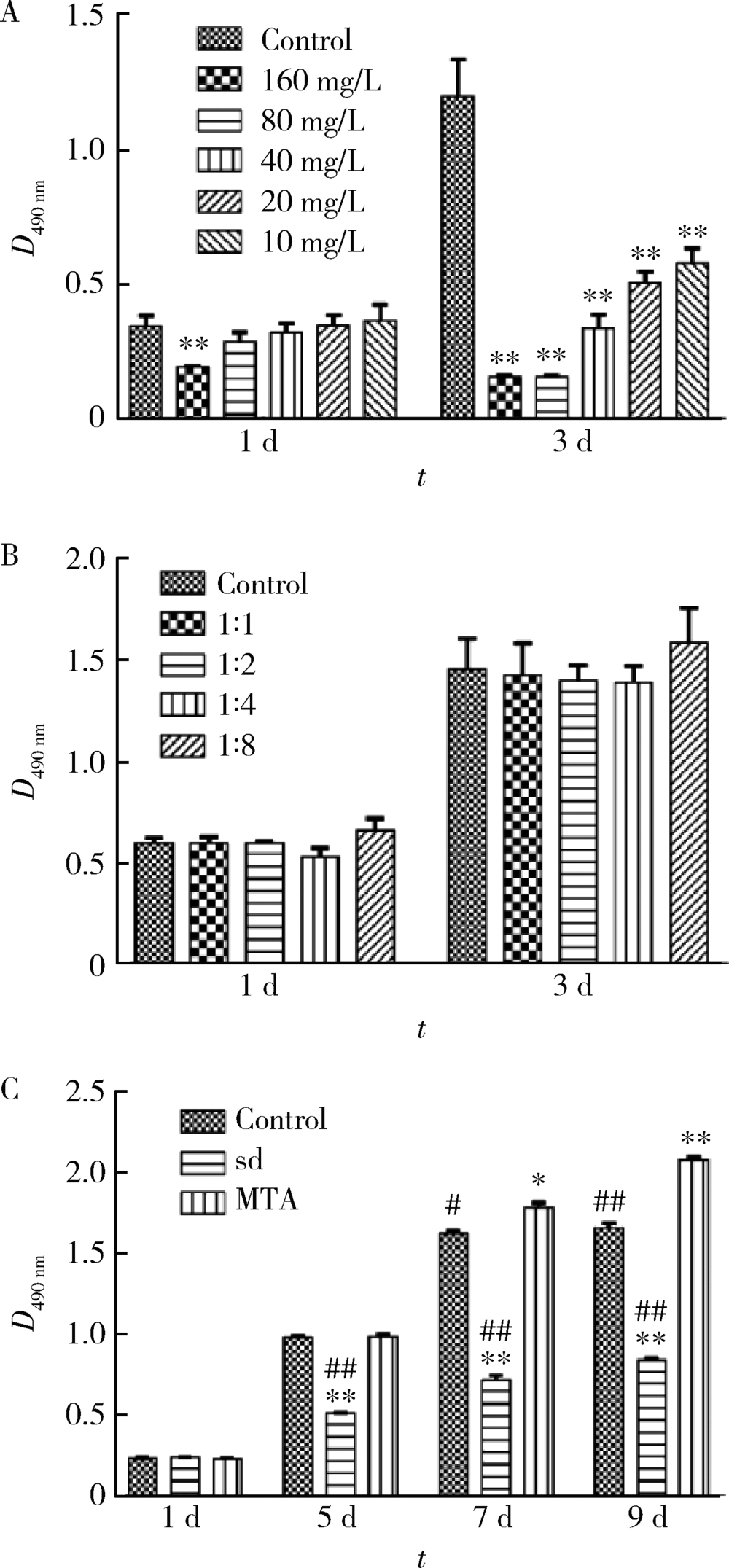
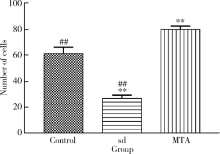
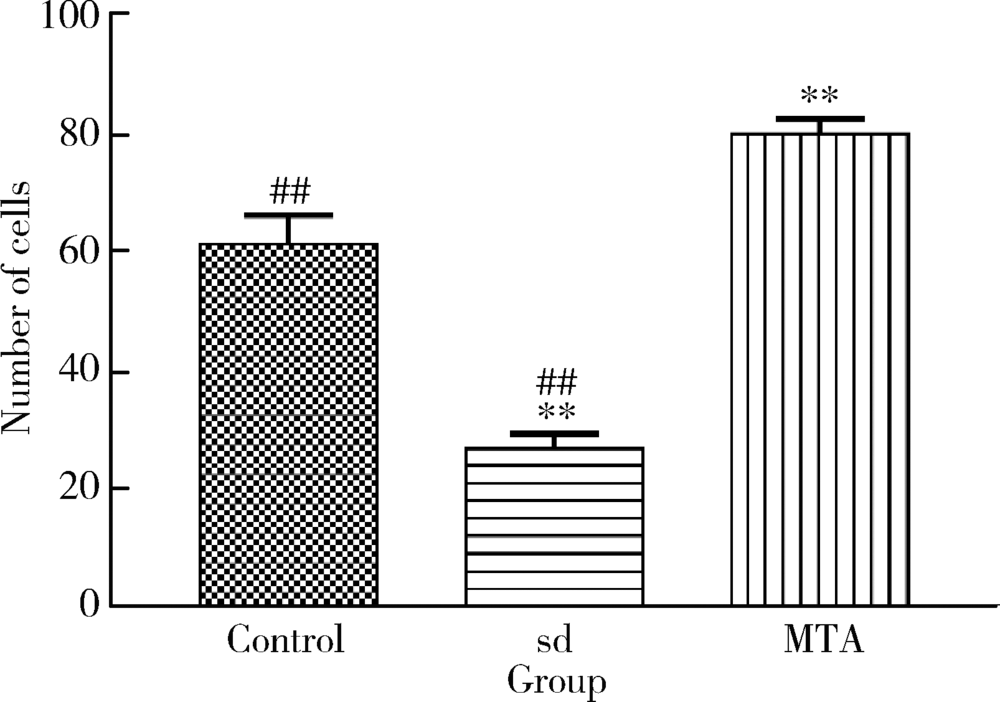
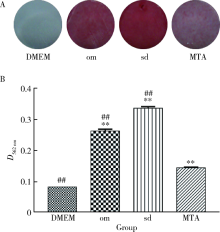
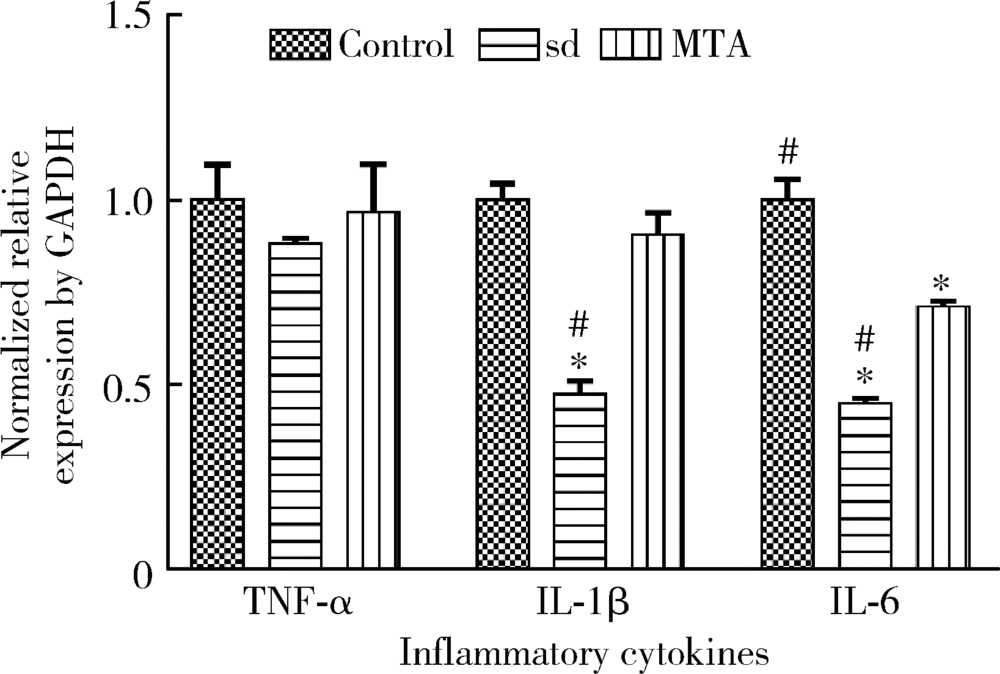
目的 比较矿物三氧化物凝聚体(mineral trioxide aggregate,MTA)与山东蜂胶乙醇提取物对牙髓成纤维细胞活性、矿化和趋化能力以及抗炎效应的影响。方法 利用CCK-8法检测山东蜂胶提取物和MTA浸出液作用牙髓成纤维细胞1、5、7、9 d时的细胞毒性,各组同时作用15 h,Transwell法检测不同组的迁移细胞数目。矿化诱导21 d后,各组进行茜素红染色比较诱导硬组织沉积能力。按照1 mg/L浓度将脂多糖(lipopolysaccharide,LPS)加入两种材料后刺激细胞3 h,采用实时聚合酶链式反应(real-time polymerase chain reaction,real-time PCR)方法检测不同组细胞肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-6(interleukin-6,IL-6)三种炎症因子的表达量。采用单因素方差分析及非参数检验对组间差异进行分析(P<0.05)。结果 蜂胶的细胞毒性大于MTA,蜂胶组迁移细胞数目(26.67±2.52)明显少于对照组(61.33±4.93)及MTA组(80.00±2.65),茜素红染色显示蜂胶组相较MTA组的钙沉积更多。LPS刺激细胞3 h后,蜂胶相比MTA可以显著降低IL-1β和IL-6的表达量。结论 山东蜂胶相比MTA对牙髓细胞有一定的细胞毒性,对细胞迁移的促进作用不明显,但蜂胶具有良好的抗炎效果及诱导牙髓细胞成牙本质分化的能力,经处理后有望应用于感染牙髓的活髓保存治疗。
中图分类号:
- R788.2
| [1] | Al-Shaher A, Wallace J, Agarwal S , et al. Effect of propolis on human fibroblasts from the pulp and periodontal ligament[J]. J Endod, 2004,30(5):359-361. |
| [2] | Al-Haj Ali SN . In vitro toxicity of propolis in comparison with other primary teeth pulpotomy agents on human fibroblasts[J]. J Investig Clin Dent, 2016,7(3):308-313. |
| [3] | Jacob A, Parolia A, Pau A , et al. The effects of Malaysian propolis and Brazilian red propolis on connective tissue fibroblasts in the wound healing process[J]. BMC Complement Altern Med, 2015,15(1):294. |
| [4] | Moradi S, Saghravanian N, Moushekhian S , et al. Immunohistochemical evaluation of fibronectin and tenascin following direct pulp capping with mineral trioxide aggregate, platelet-rich plasma and propolis in dogs’ teeth[J]. Iran Endod J, 2015,10(3):188-192. |
| [5] | Parolia A, Kundabala M, Rao N , et al. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal[J]. Aust Dent J, 2010,55(1):59-64. |
| [6] | ISO10993-12-2007医疗器械生物学评价——第12部分:样品制备与参照样品[S]. |
| [7] | Rodrigues EM, Cornélio ALG, Mestieri LB , et al. Human dental pulp cells response to MTA and MTA Plus: Cytotoxicity and gene expression analysis[J]. International Endodontic Journal, 2016,50(8):780-789. |
| [8] | Zare Jahromi M, Ranjbarian P, Shiravi S . Cytotoxicity evaluation of Iranian propolis and calcium hydroxide on dental pulp fibroblasts[J]. J Dent Res Dent Clin Dent Prospects, 2014,8(3):130-133. |
| [9] | 刘颖婷, 张玉皓 . 水溶蜂胶对人牙髓成纤维细胞的毒性作用研究[J]. 中国现代医学杂志, 2015,25(30):18-22. |
| [10] | Chang H, Wang Y, Yin X , et al. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy[J]. BMC Complement Altern Med, 2017,17(1):471. |
| [11] | Margunato S, Tasli PN, Aydin S , et al. In vitro evaluation of ProRoot MTA, Biodentine, and MM-MTA on human alveolar bone marrow stem cells in terms of biocompatibility and mineralization[J]. J Endod, 2015,41(10):1646-1652. |
| [12] | Kabala-Dzik A, Rzepecka-Stojko A, Kubina R , et al. Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: An in vitro comparison study[J]. Nutrients, 2017,9(10):1144. |
| [13] | Bueno-Silva B, Franchin M, Alves C , et al. Main pathways of action of Brazilian red propolis on the modulation of neutrophils migration in the inflammatory process[J]. Phytomedicine, 2016,23(13):1583-1590. |
| [14] | Ahangari Z, Naseri M, Jalili M , et al. Effect of propolis on dentin regeneration and the potential role of dental pulp stem cell in Guinea pigs[J]. Cell J, 2012,13(4):223-228. |
| [15] | Lima Cavendish R, de Souza Santos J, Belo Neto R , et al. Anti-nociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents[J]. J Ethnopharmacol, 2015,173:127-133. |
| [16] | das Neves MV, da Silva TM, Lima Ede O , et al. Isoflavone for-mononetin from red propolis acts as a fungicide against Candida sp.[J]. Braz J Microbiol, 2016,47(1):159-166. |
| [17] | Gemiarto AT, Ninyio NN, Lee SW , et al. Isoprenyl caffeate, a major compound in manuka propolis, is a quorum-sensing inhibitor in Chromobacterium violaceum[J]. Antonie Van Leeuwenhoek, 2015,108(2):491-504. |
| [18] | Saha S, Nair R and Asrani H. Comparative evaluation of propolis, metronidazole with chlorhexidine, calcium hydroxide and curcuma longa extract as intracanal medicament against E. faecalis: An in vitro study [J]. J Clin Diagn Res, 2015, 9(11): ZC19-21. |
| [19] | Bhandari S, Ashwini TS, Patil CR . An in vitro evaluation of antimicrobial efficacy of 2% chlorhexidine gel,propolis and calcium hydroxide against Enterococcus faecalis in human root dentin [J]. J Clin Diagn Res, 2014, 8(11): ZC60-63. |
| [20] | Neiva K, Catalfamo D, Holliday S , et al. Propolis decreases lipopolysaccharide-induced inflammatory mediators in pulp cells and osteoclasts[J]. Dent Traumatol, 2014,30(5):362-367. |
| [21] | Kusum B, Rakesh K, Richa K . Clinical and radiographical evaluation of mineral trioxide aggregate, biodentine and propolis as pulpotomy medicaments in primary teeth[J]. Restor Dent Endod, 2015,40(4):276-285. |
| [22] | Altunsoy M, Tanrıver M, Türkan U , et al. In vitro evaluation of microleakage and microhardness of ethanolic extracts of propolis in different proportions added to glass ionomer cement[J]. J Clin Pediatr Dent, 2016,40(2):136-140. |
| [23] | Prabhakar AR, Balehosur DV, Basappa N. Comparative evaluation of shear bond strength and fluoride release of conventional glass ionomer with 1% ethanolic extract of propolis incorporated glass ionomer cement: In vitro study [J]. J Clin Diagn Res, 2016, 10(5): ZC88-91. |
| [24] | Grenho L, Barros J, Ferreira C , et al. In vitro antimicrobial acti-vity and biocompatibility of propolis containing nanohydroxyapatite[J]. Biomed Mater, 2015,10(2):025004. |
| [1] | 何珊,陈炘,程琦,朱灵江,张培玉,童淑婷,薛静,杜燕. 托法替布通过JAK/STAT3通路抑制肺成纤维细胞向肌成纤维细胞转化[J]. 北京大学学报(医学版), 2024, 56(3): 505-511. |
| [2] | 赵晓一,刘畅,钱锟,潘洁. 成熟恒牙牙髓切断术的疗效及影像学评价[J]. 北京大学学报(医学版), 2024, 56(1): 138-143. |
| [3] | 郑佳佳,杨雪,温泉,付元,邵校,丁美丽. 生物活性陶瓷iRoot BP Plus®在儿童年轻恒前牙复杂冠折牙髓切断术中的应用[J]. 北京大学学报(医学版), 2024, 56(1): 179-184. |
| [4] | 叶雨阳,岳林,邹晓英,王晓燕. 成牙本质方向分化牙髓干细胞外泌体形态及微小RNA表达谱特征[J]. 北京大学学报(医学版), 2023, 55(4): 689-696. |
| [5] | 代云飞,刘鹤,彭楚芳,姜玺军. 年轻恒牙牙髓再生治疗术后36个月的临床疗效评估[J]. 北京大学学报(医学版), 2023, 55(4): 729-735. |
| [6] | 叶佳学,梁宇红. 牙髓专科医师应用锥形束CT的现况调查[J]. 北京大学学报(医学版), 2023, 55(1): 114-119. |
| [7] | 雍颹,钱锟,朱文昊,赵晓一,刘畅,潘洁. 成年恒牙牙髓切断后牙髓钙化的X线片评价[J]. 北京大学学报(医学版), 2023, 55(1): 88-93. |
| [8] | 王爽,彭楚芳,刘鹤. 新型生物陶瓷材料用于乳磨牙牙髓切断术的临床疗效[J]. 北京大学学报(医学版), 2022, 54(6): 1196-1201. |
| [9] | 蔡天玉,朱振鹏,徐纯如,吉星,吕同德,郭振可,林健. 成纤维细胞生长因子受体2在肾透明细胞癌中的表达及意义[J]. 北京大学学报(医学版), 2022, 54(4): 628-635. |
| [10] | 田靖,秦满,陈洁,夏斌. 失活剂烧伤致乳磨牙早失及恒牙胚丧失2例[J]. 北京大学学报(医学版), 2022, 54(2): 381-385. |
| [11] | 钱锟,潘洁,朱文昊,赵晓一,刘畅,雍颹. 两种硅酸钙类材料用于成熟恒牙牙髓切断术的临床效果[J]. 北京大学学报(医学版), 2022, 54(1): 113-118. |
| [12] | 郜洪宇,孟焕新,侯建霞,黄宝鑫,李玮. 钙结合蛋白在健康牙周组织和实验性牙周炎组织的表达分布[J]. 北京大学学报(医学版), 2021, 53(4): 744-749. |
| [13] | 曹春玲,杨聪翀,屈小中,韩冰,王晓燕. 可注射羟乙基壳聚糖基水凝胶理化性能及其对人牙髓细胞增殖和成牙本质向分化的作用[J]. 北京大学学报(医学版), 2020, 52(1): 10-17. |
| [14] | 陈颖怡,胡紫琪,惠甜倩,刘鹤. Zeste同源蛋白2增强子通过调节巨噬细胞趋化影响牙髓炎症反应[J]. 北京大学学报(医学版), 2020, 52(1): 18-23. |
| [15] | 李婧宜,王赛楠,董艳梅. 非甾体类抗炎药对人牙髓细胞的抗炎修复作用[J]. 北京大学学报(医学版), 2020, 52(1): 24-29. |
|
||