北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (2): 247-253. doi: 10.19723/j.issn.1671-167X.2020.02.009
肺瘤平膏联合环磷酰胺化疗对肺癌的抑瘤作用和酸性微环境的影响
- 1. 郑州大学附属肿瘤医院中西医科,郑州 450008
2. 郑州卫生健康职业学院中医教研室,郑州 450005
3. 河南中医药大学管理学院,郑州 450008
Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment
Liang GENG1,Jing LV2,△( ),Jing FAN3
),Jing FAN3
- 1. Department of Chinese and Western Medicine, Cancer Hospital Affiliated to Zhengzhou University, Zhengzhou 450008, China
2. Traditional Chinese Medicine Teaching and Research Office, Zhengzhou Health Vocational College, Zhengzhou 450005, China
3. College of Management, Henan University of Chinese Medicine, Zhengzhou 450008, China
摘要:
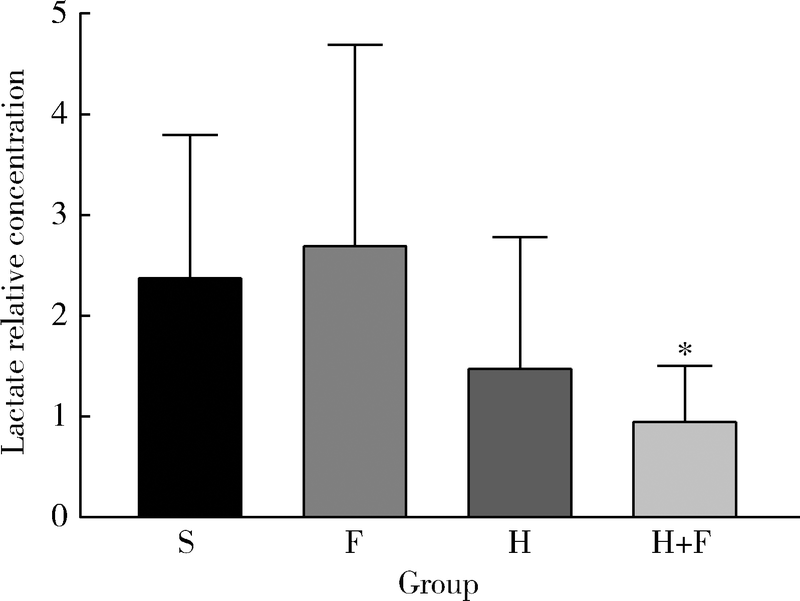
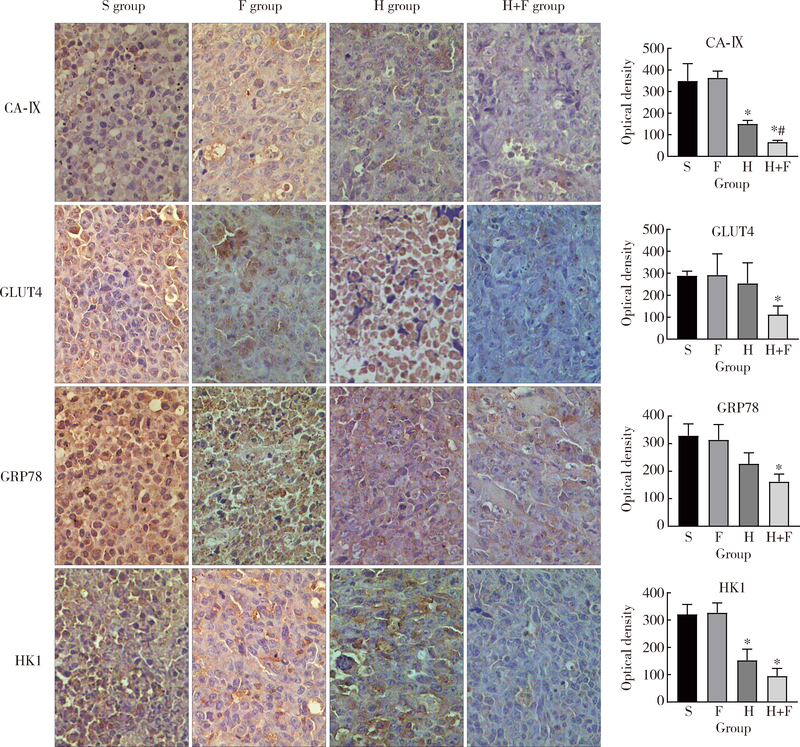
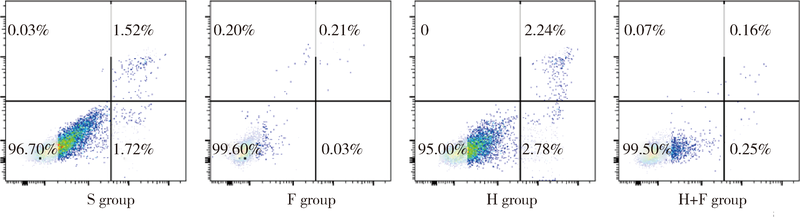
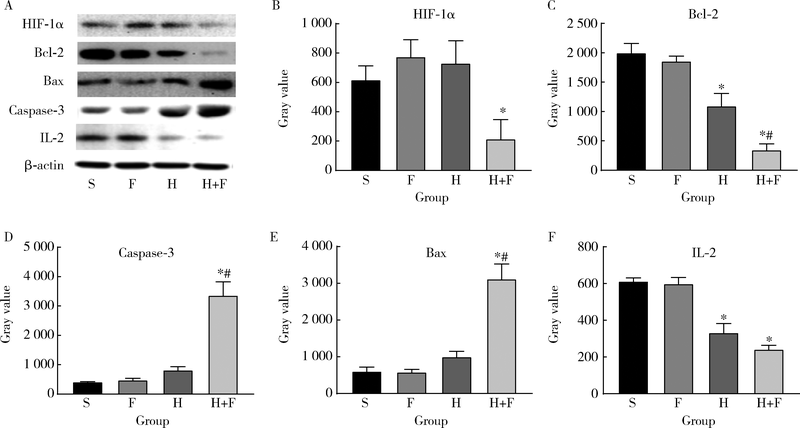
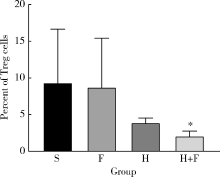
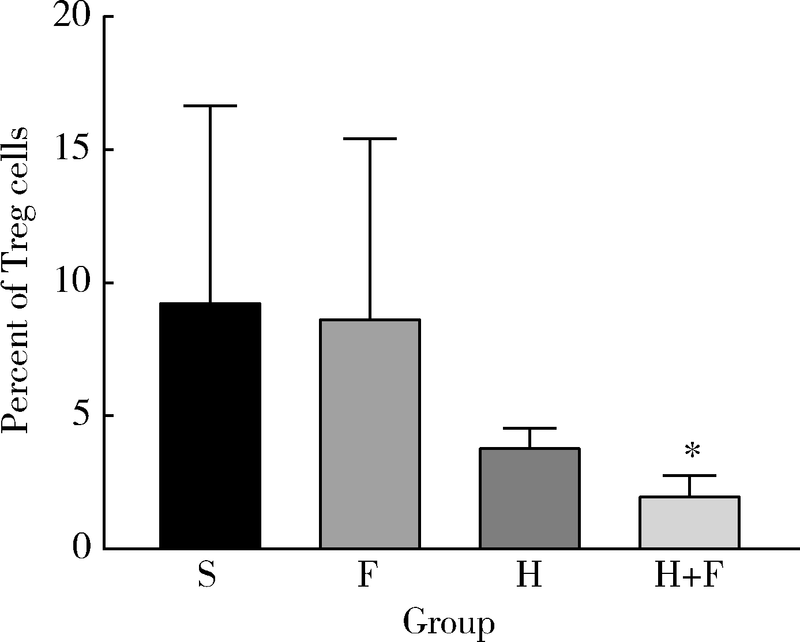
目的 观察肺瘤平膏和化疗药环磷酰胺对肺癌小鼠的作用,并从酸性微环境和细胞凋亡角度探索其内在作用机制.方法: 建立Lewis肺癌小鼠模型,分别给予环磷酰胺,肺瘤平膏,环磷酰胺+肺瘤平膏相应处理,以生理盐水组作为对照,观察各组小鼠的肿瘤体积,肿瘤增殖率,食物消耗量.给药15 d后处死小鼠,取肿瘤组织,用乳酸试剂盒检测肿瘤组织的乳酸相对浓度,免疫组织化学法检测葡萄糖转运蛋白4(glucose transporter 4,GLUT4),己糖激酶1(hexokinase 1,HK1),葡萄糖调节蛋白78(glucose-regulated protein 78,GRP78),碳酸酐酶-Ⅸ(carbonic anhydrase-Ⅸ,CA-Ⅸ)的蛋白表达,流式细胞术检测肿瘤细胞凋亡率和调节性T细胞(regulatory T cells,Treg)比例,Wes-tern blot法检测缺氧诱导因子1-α(hypoxia-inducible factor-1α,HIF-1α),Bcl-2,Bax,Caspase-3,白细胞介素2(interleukin-2,IL-2)的蛋白表达.结果: 肺瘤平膏+环磷酰胺组小鼠的肿瘤生长受到抑制最为明显,肿瘤增殖率最低,与生理盐水组相比差异有统计学意义(P<0.05),食物的消耗量也最多,但差异并不具有统计学意义(P>0.05).进一步的分子生物学检测发现,肺瘤平膏+环磷酰胺组肿瘤组织的乳酸水平在各组中最低,并伴有GLUT4,HK1,GRP78,CA-Ⅸ等蛋白表达和Treg细胞比例的下降,与生理盐水组相比差异具有统计学意义(P<0.05).此外,与生理盐水组相比,肺瘤平膏+环磷酰胺组肿瘤细胞凋亡显著增加,而HIF-1α,Bcl-2,Bax,Caspase-3,IL-2等蛋白的表达与生理盐水组相比存在明显差异.结论: 化疗药环磷酰胺与肺瘤平膏在抑制肺癌生长,提高荷瘤小鼠一般状况上具有协同作用,这些作用部分是因为二者联用改善组织缺氧,抑制HIF-1α表达,进而调控下游GLUT4,HK1,GRP78,CA-Ⅸ等蛋白的表达,降低肿瘤组织局部的乳酸水平,改善肿瘤组织酸性微环境,从而诱导肿瘤细胞凋亡,抑制T细胞向Treg细胞分化.
中图分类号:
- R734.2
| [1] | 陈璐, 高威 . 肿瘤酸性微环境的形成机制及其对肿瘤进展的影响[J]. 肿瘤, 2019,39(2):140-145. |
| [2] | Li X, Yu X, Dai D , et al. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters[J]. Oncotarget, 2016,7(17):23141-23155. |
| [3] | Wojtkowiak JW, Verduzco D, Schramm KJ , et al. Drug resistance and cellular adaptation to tumor acidic pH microenvironment[J]. Mol Pharm, 2011,8(6):2032-2038. |
| [4] | 龚巍 . 调节性T细胞和胃癌的临床与实验研究[D]. 苏州: 苏州大学, 2017. |
| [5] | Fukumura D, Jain RK . Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization[J]. Microvasc Res, 2007,74(2-3):72-84. |
| [6] | Ganapathy V, Thangaraju M, Prasad PD . Nutrient transporters in cancer: Relevance to Warburg hypojournal and beyond[J]. Pharmacol Ther, 2009,121(1):29-40. |
| [7] | Seagroves TN, Ryan HE, Lu H , et al. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells[J]. Mol Cell Biol, 2001,21(10):3436-3444. |
| [8] | Semenza GL . Targeting HIF-1 for cancer therapy[J]. Nat Rev Cancer, 2003,3(10):721-732. |
| [9] | Lee JW, Bae SH, Jeong JW , et al. Hypoxia-inducible factor (HIF-1) alpha: Its protein stability and biological functions[J]. Exp Mol Med, 2004,36(1):1-12. |
| [10] | Winum JY, Rami M, Scozzafava A , et al. Carbonic anhydrase Ⅸ: A new druggable target for the design of antitumor agents[J]. Med Res Rev, 2008,28(3):445-463. |
| [11] | van den Beucken T, Ramaekers CH, Rouschop K , et al. Deficient carbonic anhydrase 9 expression in UPR-impaired cells is associated with reduced survival in an acidic microenvironment[J]. Radiother Oncol, 2009,92(3):437-442. |
| [12] | Verras M, Papandreou I, Lim AL , et al. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress[J]. Mol Cell Biol, 2008,28(23):7212-7224. |
| [13] | 张翔云, 周华妙, 郭勇 . 黄连解毒汤等对乳腺癌荷瘤小鼠肿瘤酸性微环境pH值的影响[J]. 黑龙江中医药, 2016,45(4):64-65. |
| [14] | Flinck M, Kramer SH, Pedersen SF . Roles of pH in control of cell proliferation[J]. Acta Physiol (Oxf), 2018,223(3):e13068. |
| [15] | Peppicelli S, Andreucci E, Ruzzolini J . The acidic microenvironment as a possible niche of dormant tumor cells[J]. Cell Mol Life Sci, 2017,74(15):2761-2771. |
| [16] | Ryder C , McColl K,Zhong F, et al.Acidosis promotes Bcl-2 family-mediated evasion of apoptosis: Involvement of acid-sensing G protein-coupled receptor Gpr65 signaling to Mek/Erk[J]. J Biol Chem, 2012,287(33):27863-27867. |
| [17] | Dhup S, Dadhich RK, Porporato PE , et al. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis[J]. Curr Pharm Des, 2012,18(10):1319-1330. |
| [1] | 柴晓东,孙子文,李海爽,朱靓怡,刘小旦,刘延涛,裴斐,常青. 髓母细胞瘤分子亚型中CD8+T淋巴细胞浸润的临床病理特点[J]. 北京大学学报(医学版), 2024, 56(3): 512-518. |
| [2] | 刘耘充,吴宗龙,葛力源,杜坦,吴雅倩,宋一萌,刘承,马潞林. 肾透明细胞癌中核蛋白1对阿昔替尼耐药的作用及机制[J]. 北京大学学报(医学版), 2023, 55(5): 781-792. |
| [3] | 刘京,陆爱东,左英熹,吴珺,黄志卓,贾月萍,丁明明,张乐萍,秦炯. 儿童急性淋巴细胞白血病合并癫痫发作75例临床特征和预后分析[J]. 北京大学学报(医学版), 2022, 54(5): 948-953. |
| [4] | 顾阳春,刘颖,谢超,曹宝山. 程序性死亡蛋白-1抑制剂治疗晚期肺癌出现垂体免疫不良反应3例[J]. 北京大学学报(医学版), 2022, 54(2): 369-375. |
| [5] | 廖栩鹤,王荣福,刘萌,陈雪祺,熊焰,农琳,殷雷,张炳晔,杜毓菁. 18F-FDG PET/CT半定量参数、表皮生长因子受体和间变淋巴瘤激酶基因突变对肺腺癌患者预后评估的价值[J]. 北京大学学报(医学版), 2021, 53(2): 246-254. |
| [6] | 鲍轶,莫娟芬. 同时性多原发肺腺癌组织编码转录因子ERG基因相同位点突变1例报告[J]. 北京大学学报(医学版), 2020, 52(5): 971-974. |
| [7] | 孙志伟,贾军,杨颖,刘传玲,肖艳洁,余靖,张晓东. 肠内营养支持治疗减轻晚期食管癌患者化疗不良反应[J]. 北京大学学报(医学版), 2020, 52(2): 261-268. |
| [8] | 马义祥,刘敬伟,齐康,张继新,林钢,刘海波,商学谦,李简. 原发性纵隔卵黄囊瘤7例[J]. 北京大学学报(医学版), 2019, 51(6): 1091-1095. |
| [9] | 王昊,陈亮,叶小云. 雷公藤甲素对TM4细胞氧化应激及PI3K/AKT通路的影响[J]. 北京大学学报(医学版), 2018, 50(4): 607-612. |
| [10] | 梅放,赵婷婷,高菲,郑杰. 肺罕见良性双相分化性肿瘤——肺腺纤维瘤1例并文献复习[J]. 北京大学学报(医学版), 2017, 49(6): 1076-1080. |
| [11] | 陈杨,王艳荣,石燕,戴广海. 晚期结直肠癌患者一线应用FOLFOX方案化疗引起中性粒细胞减少的预后价值[J]. 北京大学学报(医学版), 2017, 49(4): 669-674. |
| [12] | 方伟岗,田新霞. 肿瘤微环境中一种新型促侵袭因子的发现——细胞外ATP功能及机制的研究进展[J]. 北京大学学报(医学版), 2017, 49(2): 188-195. |
| [13] | 王玉洁,郭向阳,王军. 重复异丙酚麻醉对新生大鼠海马细胞凋亡及远期学习记忆能力的影响[J]. 北京大学学报(医学版), 2017, 49(2): 310-314. |
| [14] | 杨光,程庆砾,李春霖,贾雅丽,岳文,裴雪涛,刘洋,赵佳慧,杜婧,敖强国. 高糖减弱肾组织干细胞条件培养液对缺氧损伤肾小管上皮细胞的修复作用[J]. 北京大学学报(医学版), 2017, 49(1): 125-130. |
| [15] | 曹珮,姜学军,席志军. 舒尼替尼通过抑制Akt/mTOR信号通路诱导肾癌细胞自噬[J]. 北京大学学报(医学版), 2016, 48(4): 584-589. |
|
||