北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (2): 254-260. doi: 10.19723/j.issn.1671-167X.2020.02.010
真实世界吡咯替尼治疗HER2阳性转移性乳腺癌的疗效及安全性
宋国红1,李惠平1,△( ),邸立军1,严颖1,姜晗昉1,徐玲2,万冬桂3,李瑛4,王墨培5,肖宇5,张如艳1,冉然1,王环1
),邸立军1,严颖1,姜晗昉1,徐玲2,万冬桂3,李瑛4,王墨培5,肖宇5,张如艳1,冉然1,王环1
- 1. 北京大学肿瘤医院暨北京市肿瘤防治研究所乳腺肿瘤内科, 恶性肿瘤发病机制及转化研究教育部重点实验室, 北京 100142
2. 北京大学第一医院乳腺疾病中心, 北京 100034
3. 中日友好医院中西医结合肿瘤科, 北京 100029
4. 中国人民解放军总医院肿瘤内科,北京 100853
5. 北京大学第三医院肿瘤化疗与放射病科, 北京 100191
Efficacy and safety of oral pyrotinib in HER2 positive metastatic breast cancer: real-world practice
Guo-hong SONG1,Hui-ping LI1,△( ),Li-jun DI1,Ying YAN1,Han-fang JIANG1,Ling XU2,Dong-gui WAN3,Ying LI4,Mo-pei WANG5,Yu XIAO5,Ru-yan ZHANG1,Ran RAN1,Huan WANG1
),Li-jun DI1,Ying YAN1,Han-fang JIANG1,Ling XU2,Dong-gui WAN3,Ying LI4,Mo-pei WANG5,Yu XIAO5,Ru-yan ZHANG1,Ran RAN1,Huan WANG1
- 1. Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Department of Breast Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
2. Breast Disease Center, Peking University First Hospital, Beijing 100034, China
3. Department of TCM Oncology, China-Japan Friendship Hospital, Beijing 100029, China
4. Department of Oncology, General Hospital of PLA, Beijing 100853, China
5. Department of Tumor Chemotherapy and Radiation Sickness, Peking University Third Hospital, Beijing 100191, China
摘要:
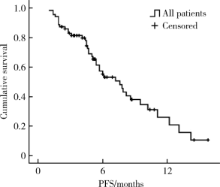
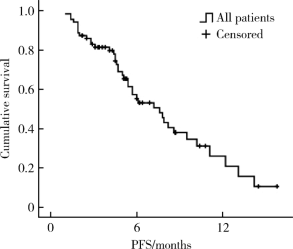
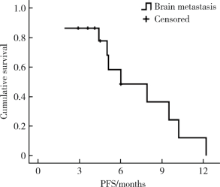
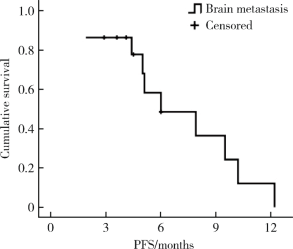
目的 评价真实世界中口服吡咯替尼治疗表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)阳性转移性乳腺癌的疗效和毒副反应.方法: 回顾性分析72例接受以口服吡咯替尼为治疗基础的HER2阳性转移性乳腺癌患者.结果: 72例HER2阳性转移性乳腺癌患者中69例(95.8%)在复发转移阶段和/或(新)辅助治疗阶段曾行抗HER2靶向治疗;61例(84.7%)在复发转移阶段接受过抗HER2靶向治疗药物,包括曲妥珠单抗56例(77.8%),拉帕替尼36例(50.0%),T-DM1 4例(5.6%).72例患者中接受吡咯替尼联合化疗(±曲妥珠单抗)62例(86.1%),吡咯替尼联合内分泌治疗(±曲妥珠单抗)6例(8.3%),吡咯替尼(±曲妥珠单抗)4例(5.6%).72例患者均可评价疗效,其中完全缓解1例(1.4%),部分缓解18例(25.0%),疾病稳定41例(56.9%),疾病进展12例(16.7%).客观缓解率(完全缓解+部分缓解)为26.4%,中位无进展生存期(progression free survival, PFS)为7.6个月(95%CI: 5.5~9.7个月).36例曾接受过拉帕替尼治疗的患者中,吡咯替尼治疗的中位PFS为7.9个月(95%CI: 4.1~11.7个月), 15例脑转移患者中,吡咯替尼治疗的中位PFS为6.0个月(95%CI: 2.2~9.8个月).吡咯替尼相关的主要毒副反应为腹泻,共57例(79.2%),1~2级者48例(66.7%),3级者9例(12.5%).结论: 以吡咯替尼为基础的方案能够有效治疗HER2阳性转移性乳腺癌,包括拉帕替尼治疗失败及脑转移的患者,不良反应可耐受.
中图分类号:
- R737.9
| [1] | Slamon DJ, Clark GM, Wong SG , et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene[J]. Science, 1987,235(4785):177-182. |
| [2] | Xu B, Hu X, Zheng H , et al. Outcomes of re-treatment with first-line trastuzumab plus a taxane in HER2 positive metastatic breast cancer patients after (neo) adjuvant trastuzumab: A prospective multicenter study[J]. Oncotarget, 2016,7(31):50643-50655. |
| [3] | Cobleigh M, Yardley D, Brufsky AM , et al. Baseline characteristics, treatment patterns, and outcomes in patients with HER2-positive metastatic breast cancer by hormone receptor status from SystHERs[J]. Clin Cancer Res, 2020,26(5):1105-1113. |
| [4] | Ma F, Li Q, Chen S , et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer[J]. J Clin Oncol, 2017,35(27):3105-3112. |
| [5] | Li Q, Guan X, Chen S , et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase Ⅰ clinical trial[J]. Clin Cancer Res, 2019,25(17):5212-5220. |
| [6] | Ma F, Ouyang Q, Li W , et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase Ⅱ study[J]. J Clin Oncol, 2019,37(29):2610-2619. |
| [7] | Jiang ZF, Yan M, Hu XC , et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: A randomized phase Ⅲ study[J]. J Clin Oncol, 2019,37(15 suppl):1001. |
| [8] | Wong H, Leung R, Kwong A , et al. Integrating molecular mechanisms and clinical evidence in the management of trastuzumab resistant or refractory HER-2 + metastatic breast cancer [J]. Oncologist, 2011,16(11):1535-1546. |
| [9] | Dawood S, Broglio K, Buzdar AU , et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review[J]. J Clin Oncol, 2010,28(1):92-98. |
| [10] | Slamon DJ, Leyland-Jones B, Shak S , et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J]. N Engl J Med, 2001,344(11):783-792. |
| [11] | Burstein HJ, Keshaviah A, Baron AD , et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study[J]. Cancer, 2007,110(5):965-972. |
| [12] | Robert N, Leyland-Jones B, Asmar L , et al. Randomized phase Ⅲ study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpres-sing metastatic breast cancer[J]. J Clin Oncol, 2006,24(18):2786-2792. |
| [13] | Geyer CE, Forster J, Lindquist D , et al. Lapatinib plus capeci-tabine for HER2-positive advanced breast cancer[J]. N Engl J Med, 2006,355(26):2733-2743. |
| [14] | Cameron D, Casey M, Oliva C , et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase Ⅲ randomized trial[J]. Oncologist, 2010,15(9):924-934. |
| [15] | Xu BH, Jiang ZF, Chua D , et al. Lapatinib plus capecitabine in treating HER2-positive advanced breast cancer: efficacy, safety, and biomarker results from Chinese patients[J]. Chin J Cancer, 2011,30(5):327-335. |
| [16] | Baselga J, Cortés J, Kim SB , Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer[J]. N Engl J Med, 2012,366(2):109-119. |
| [17] | Verma S, Miles D, Gianni L , et al. Trastuzumab emtansine for HER2-positive advanced breast cancer[J]. N Engl J Med, 2012,367(19):1783-1791. |
| [18] | Leyland-Jones B . Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases[J]. J Clin Oncol, 2009,27(31):5278-5286. |
| [19] | Ramakrishna N, Temin S, Chandarlapaty S , et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline[J]. J Clin Oncol, 2014,32(19):2100-2108. |
| [20] | Ramakrishna N, Temin S, Chandarlapaty S , et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO clinical practice guideline update[J]. J Clin Oncol, 2018,36(27):2804-2807. |
| [21] | Bachelot T, Romieu G, Campone M , et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study[J]. Lancet Oncol, 2013,14(1):64-71. |
| [22] | Montemurro F, Ellis P, Delaloge S, et al. Safety and efficacy of trastuzumab emtansine(>T-DM1) in 399 patients with central nervous system metastases: Exploratory subgroup analysis from the KAMILLA study[J].Cancer Res, 2017, 77(4 Suppl):P1-12-10. |
| [1] | 汤莹, 张湧波, 吴丹红, 林炎鸿, 兰风华. 13例先天性双侧输精管缺如不育患者的致病基因突变检测[J]. 北京大学学报(医学版), 2024, 56(5): 763-774. |
| [2] | 扶琼,叶霜. 嵌合抗原受体T细胞治疗在自身免疫疾病中的应用和思考[J]. 北京大学学报(医学版), 2023, 55(6): 953-957. |
| [3] | 马建勋,夏有辰,李比,赵红梅,雷玉涛,布希. 乳腺癌改良根治术后即刻乳房重建的方式选择[J]. 北京大学学报(医学版), 2023, 55(4): 612-618. |
| [4] | 张云静,乔丽颖,祁萌,严颖,亢伟伟,刘国臻,王明远,席云峰,王胜锋. 乳腺癌患者新发心血管疾病预测模型的建立与验证:基于内蒙古区域医疗数据[J]. 北京大学学报(医学版), 2023, 55(3): 471-479. |
| [5] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [6] | 程昉,杨邵英,房星星,王璇,赵福涛. CCL28-CCR10通路在类风湿关节炎单核细胞迁移中的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1074-1078. |
| [7] | 董尔丹. 心血管受体的信号转导与疾病[J]. 北京大学学报(医学版), 2022, 54(5): 796-802. |
| [8] | 王跃,张爽,张虹,梁丽,徐玲,程元甲,段学宁,刘荫华,李挺. 激素受体阳性/人表皮生长因子受体2阴性乳腺癌临床病理特征及预后[J]. 北京大学学报(医学版), 2022, 54(5): 853-862. |
| [9] | 程晓静,蒋栋,张连海,王江华,李雅真,翟佳慧,闫宝琪,张露露,谢兴旺,李子禹,季加孚. KRAS G12V特异性T细胞受体治疗恶性肿瘤的临床前研究[J]. 北京大学学报(医学版), 2022, 54(5): 884-895. |
| [10] | 李芷晴,俞冰,蔡泽宇,王迎宝,张煦,周彪,方晓红,于芳,付毅,孙金鹏,李伟,孔炜. 柚皮素抑制马凡综合征小鼠胸主动脉瘤的形成[J]. 北京大学学报(医学版), 2022, 54(5): 896-906. |
| [11] | 蔡天玉,朱振鹏,徐纯如,吉星,吕同德,郭振可,林健. 成纤维细胞生长因子受体2在肾透明细胞癌中的表达及意义[J]. 北京大学学报(医学版), 2022, 54(4): 628-635. |
| [12] | 顾阳春,刘颖,谢超,曹宝山. 程序性死亡蛋白-1抑制剂治疗晚期肺癌出现垂体免疫不良反应3例[J]. 北京大学学报(医学版), 2022, 54(2): 369-375. |
| [13] | 田佳宜,张霞,程功,刘庆红,王世阳,何菁. 系统性红斑狼疮患者血清白细胞介素-2受体α水平及其临床意义[J]. 北京大学学报(医学版), 2021, 53(6): 1083-1087. |
| [14] | 廖栩鹤,王荣福,刘萌,陈雪祺,熊焰,农琳,殷雷,张炳晔,杜毓菁. 18F-FDG PET/CT半定量参数、表皮生长因子受体和间变淋巴瘤激酶基因突变对肺腺癌患者预后评估的价值[J]. 北京大学学报(医学版), 2021, 53(2): 246-254. |
| [15] | 张晓鹏,张维宇,霍飞,胡浩,王起,许克新. 原发性女性膀胱出口梗阻的手术疗效随访及其发病机制[J]. 北京大学学报(医学版), 2019, 51(6): 1052-1055. |
|
||