北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (4): 628-635. doi: 10.19723/j.issn.1671-167X.2022.04.008
成纤维细胞生长因子受体2在肾透明细胞癌中的表达及意义
- 北京大学第一医院泌尿外科,北京大学泌尿外科研究所,国家泌尿男生殖系肿瘤研究中心,北京 100034
Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma
Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN*( )
)
- Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Can-cer Center, Beijing 100034, China
摘要:
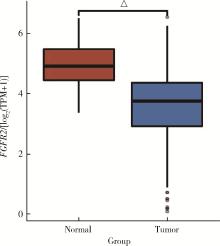
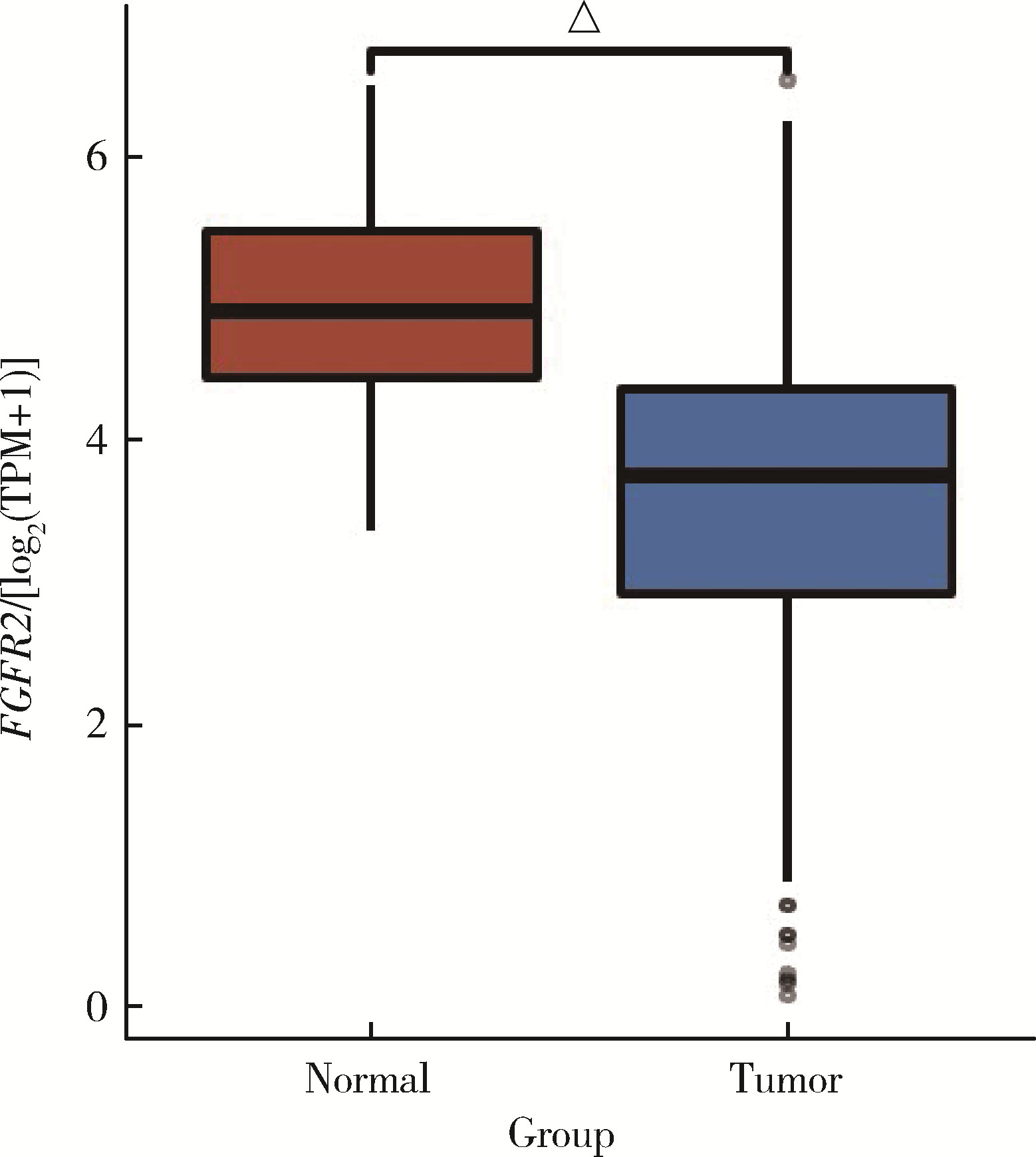
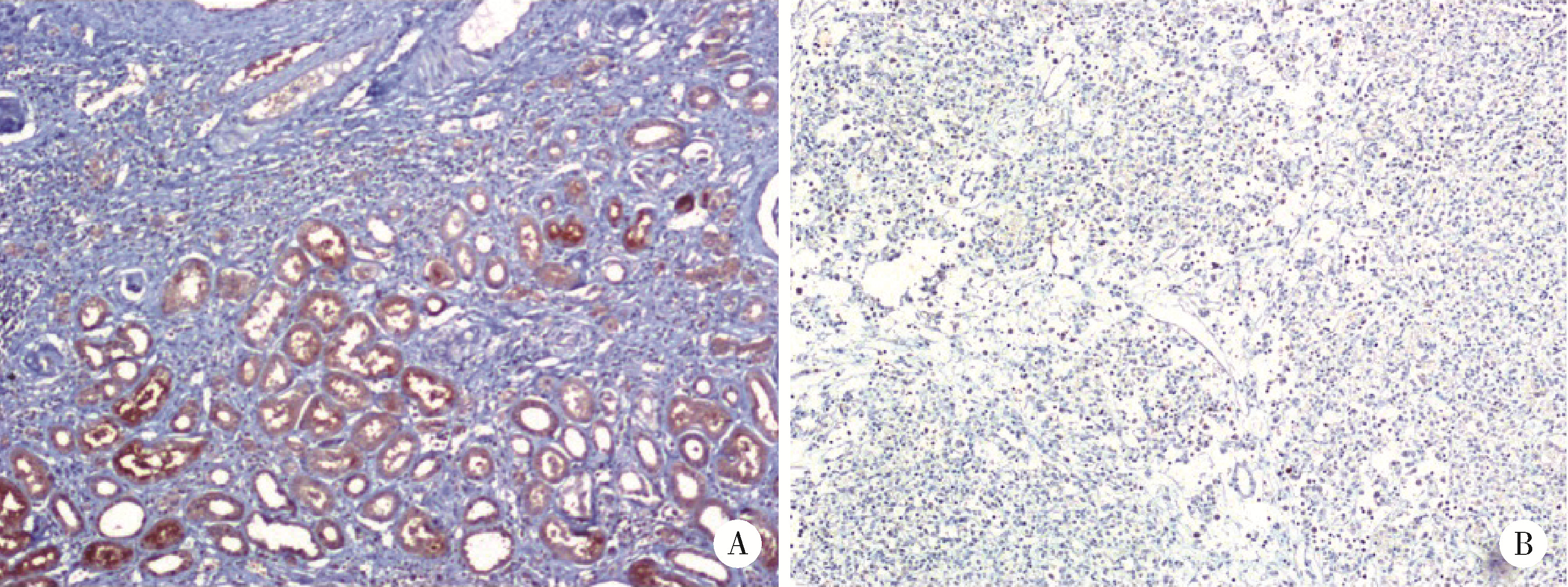
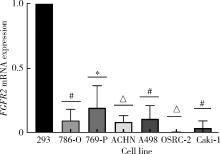
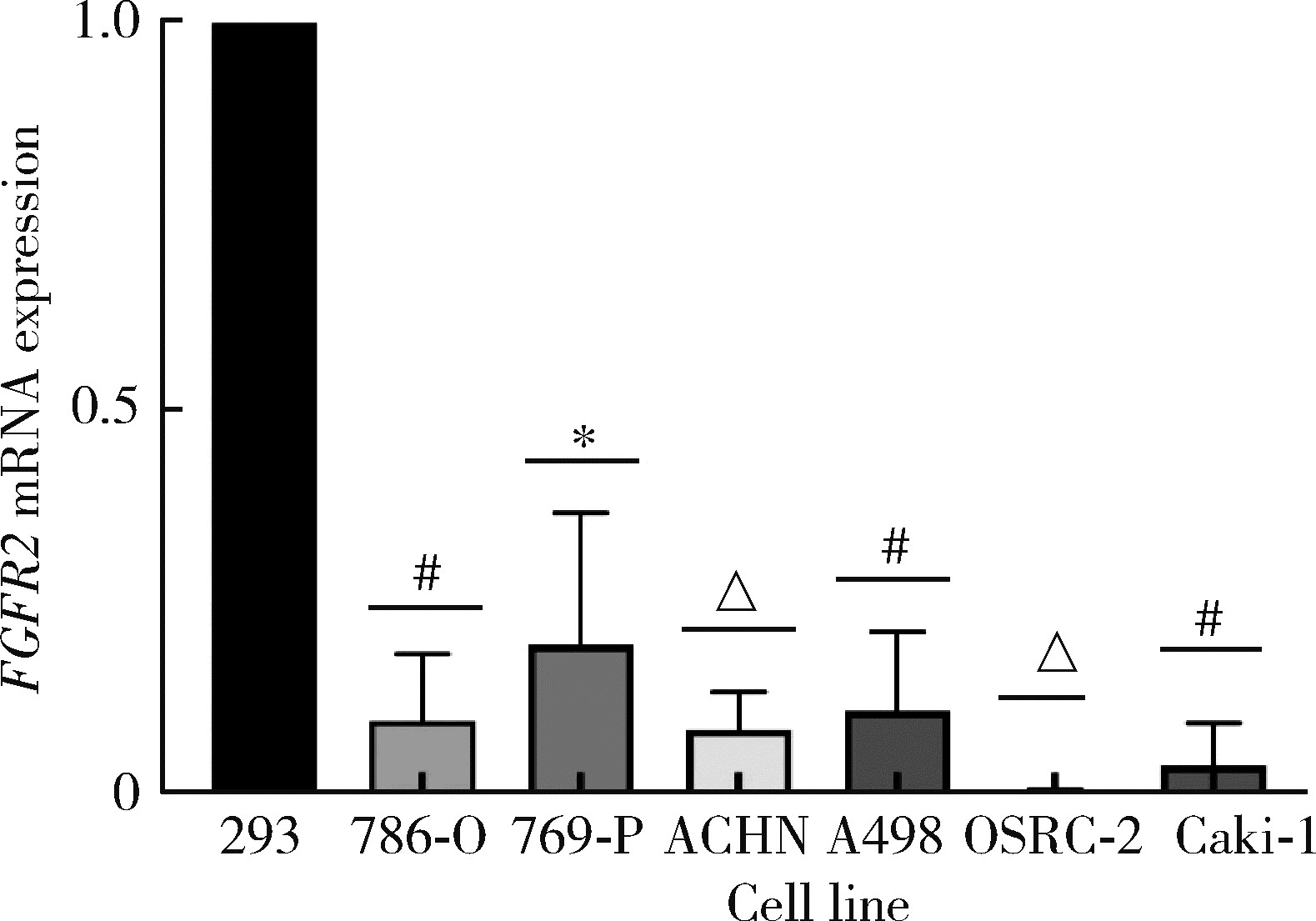
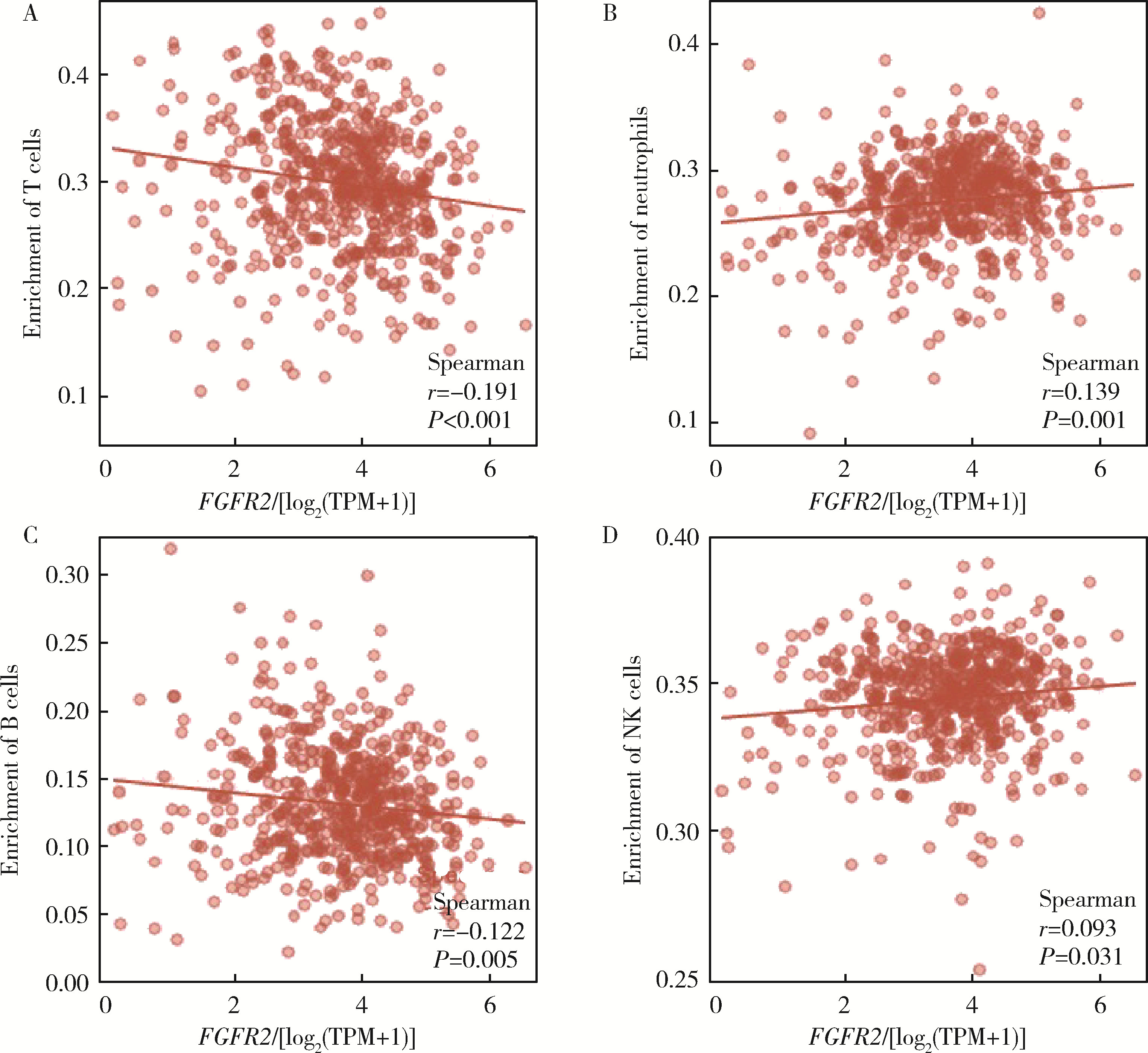
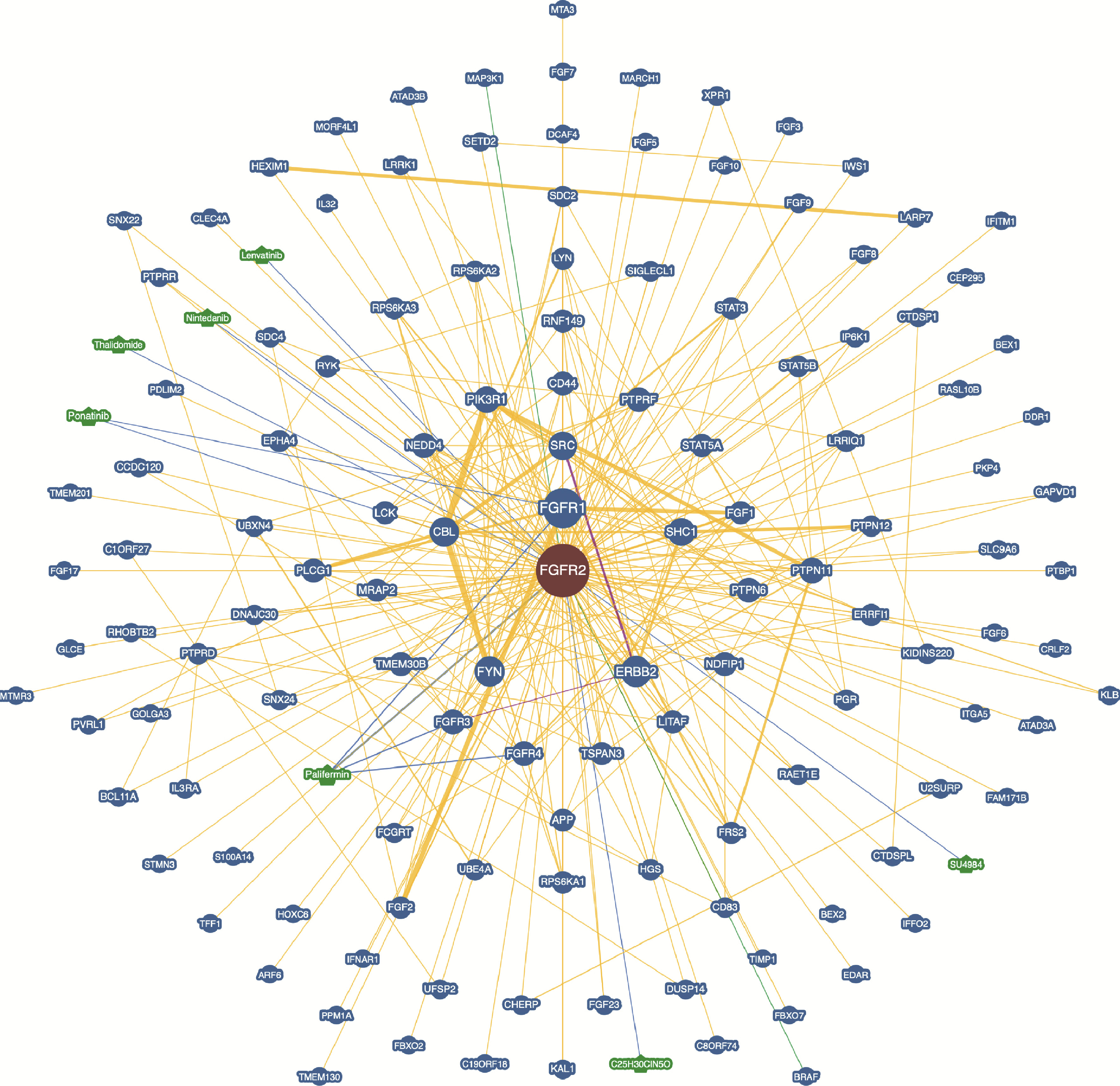
目的: 研究成纤维细胞生长因子受体2(fibroblast growth factor receptor 2,FGFR2)在肾透明细胞癌(clear cell renal cell carcinoma,ccRCC;or kidney renal clear cell carcinoma,KIRC)中的表达情况,对FGFR2的表达与ccRCC临床病理特征及预后关系进行分析,并研究FGFR2的表达与其他分子之间作用关系,探讨其在ccRCC发生发展中的作用。方法: 从肿瘤基因图谱(The Cancer Genome Atlas, TCGA)数据库及基因表达综合(Gene Expression Omnibus,GEO)数据库中下载ccRCC患者的基因表达及临床信息资料,进行转化及整理,并收集北京大学第一医院泌尿外科104例临床ccRCC样本及癌旁正常组织样本,进行免疫组织化学染色(immunohistochemistry, IHC),对染色结果进行评分,从而比较ccRCC和癌旁正常组织中FGFR2的蛋白表达情况;采用实时荧光定量聚合酶链反应(quantify real-time polymerase chain reaction,qRT-PCR)检测正常的肾上皮细胞系(293)和肾细胞癌细胞系(786-O、769-P、OSRC-2、Caki-1、ACHN、A498)中FGFR2的mRNA表达水平;对数据库中的ccRCC患者进行进一步分析,研究FGFR2表达与ccRCC患者临床病理特征(包括TNM分期和病理分级)以及生存预后的关系,分析ccRCC患者中FGFR2表达与B细胞、T细胞、自然杀伤(natural killer,NK)细胞及中性粒细胞浸润的关系,并使用生物交互数据集通用存储库(Biological General Repository for Interactionh Datasets,BioGRID)构建蛋白质相互作用(protein-protein interaction,PPI)网络,利用网络研究与FGFR2蛋白相互作用的分子。结果: 在TCGA数据库中,相较于正常组织样本,FGFR2在ccRCC组织样本中表达下调,在GEO数据库中的表达也呈现出这一差异,并且FGFR2在ccRCC临床样本及ccRCC细胞系中表达下调,此外,FGFR2表达水平与ccRCC高分级分期相关,与ccRCC患者较好的预后相关,并且FGFR2表达与B细胞、T细胞、NK细胞及中性粒细胞浸润无明显相关关系。PPI网络显示FGFR2蛋白与某些分子间存在相互作用。结论: 本研究揭示了FGFR2与ccRCC发生发展的潜在作用关系,提示FGFR2可能作为ccRCC患者的预后标志物和潜在治疗靶点。
中图分类号:
- R34
| 1 |
Cohen HT . Renal-cell carcinoma[J]. N Engl J Med, 2005, 353 (23): 2477- 2490.
doi: 10.1056/NEJMra043172 |
| 2 |
Siegel RL , Miller KD , Fuchs HE , et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71 (1): 7- 33.
doi: 10.3322/caac.21654 |
| 3 |
Hsieh JJ , Purdue MP , Signoretti S , et al. Renal cell carcinoma[J]. Nat Rev Dis Primers, 2017, 3 (1): 17009.
doi: 10.1038/nrdp.2017.9 |
| 4 |
Rini BI , Campbell SC , Escudier B . Renal cell carcinoma[J]. Lancet, 2009, 373 (9669): 1119- 1132.
doi: 10.1016/S0140-6736(09)60229-4 |
| 5 | Penticuff JC , Kyprianou N . Therapeutic challenges in renal cell carcinoma[J]. Am J Clin Exp Urol, 2015, 3 (2): 77- 90. |
| 6 |
Wolff I , May M , Hoschke B , et al. Do we need new high-risk criteria for surgically treated renal cancer patients to improve the outcome of future clinical trials in the adjuvant setting? Results of a comprehensive analysis based on the multicenter CORONA database[J]. Eur J Surg Oncol, 2016, 42 (5): 744- 750.
doi: 10.1016/j.ejso.2016.01.009 |
| 7 |
Williamson TJ , Pearson JR , Ischia J , et al. Guideline of guidelines: follow-up after nephrectomy for renal cell carcinoma[J]. BJU Int, 2016, 117 (4): 555- 562.
doi: 10.1111/bju.13384 |
| 8 |
Eswarakumar VP , Lax I , Schlessinger J . Cellular signaling by fibroblast growth factor receptors[J]. Cytokine Growth Factor Rev, 2005, 16 (2): 139- 149.
doi: 10.1016/j.cytogfr.2005.01.001 |
| 9 |
Li P , Huang T , Zou Q , et al. FGFR2 promotes expression of PD-L1 in colorectal cancer via the JAK/STAT3 signaling pathway[J]. J Immunol, 2019, 202 (10): 3065- 3075.
doi: 10.4049/jimmunol.1801199 |
| 10 |
Wang Y , Shi T , Wang X , et al. FGFR2 alteration as a potential therapeutic target in poorly cohesive gastric carcinoma[J]. J Transl Med, 2021, 19 (1): 1- 11.
doi: 10.1186/s12967-020-02683-4 |
| 11 |
Oughtred R , Rust J , Chang C , et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions[J]. Protein Sci, 2021, 30 (1): 187- 200.
doi: 10.1002/pro.3978 |
| 12 |
Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70 (1): 7- 30.
doi: 10.3322/caac.21590 |
| 13 | Turner N , Grose R . Fibroblast growth factor signalling: from development to cancer[J]. Nat Rev Cancer, 2010, 10 (2): 116- 129. |
| 14 |
Zhang J , Wong CC , Leung KT , et al. FGF18-FGFR2 signaling triggers the activation of c-Jun-YAP1 axis to promote carcinoge-nesis in a subgroup of gastric cancer patients and indicates translational potential[J]. Oncogene, 2020, 39 (43): 6647- 6663.
doi: 10.1038/s41388-020-01458-x |
| 15 |
Huang T , Liu D , Wang Y , et al. FGFR2 Promotes gastric cancer progression by inhibiting the expression of thrombospondin4 via PI3K-Akt-Mtor pathway[J]. Cell Physiol Biochem, 2018, 50 (4): 1332- 1345.
doi: 10.1159/000494590 |
| 16 |
Lei JH , Lee MH , Miao K , et al. Activation of FGFR2 signaling suppresses BRCA1 and drives triple-negative mammary tumorigenesis that is sensitive to immunotherapy[J]. Adv Sci, 2021, 8 (21): e2100974.
doi: 10.1002/advs.202100974 |
| 17 | Goyal L , Shi L , Liu LY , et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma[J]. Cancer Discov, 2019, 9 (8): 1064- 1079. |
| 18 |
Lee JE , Shin SH , Shin HW , et al. Nuclear FGFR2 negatively regulates hypoxia-induced cell invasion in prostate cancer by interacting with HIF-1 and HIF-2[J]. Sci Rep, 2019, 9 (1): 3480.
doi: 10.1038/s41598-019-39843-6 |
| 19 | Volkova M , Tsimafeyeu I , Olshanskaya A , et al. Immunochemical expression of fibroblast growth factor and its receptors in primary tumor cells of renal cell carcinoma[J]. Am J Clin Exp Urol, 2021, 9 (1): 65- 72. |
| 20 | Zhao Q , Caballero OL , Davis ID , et al. Tumor-specific isoform switch of the fibroblast growth factor receptor 2 underlies the mesenchymal and malignant phenotypes of clear cell renal cell carcinomas[J]. Clin Cancer Res, 2013, 19 (9): 2460- 2472. |
| 21 | Ranieri D , Nanni M , Guttieri L , et al. The aberrant expression in epithelial cells of the mesenchymal isoform of FGFR2 controls the negative crosstalk between EMT and autophagy[J]. J Cell Mol Med, 2021, 25 (8): 4166- 4172. |
| [1] | 刘耘充,吴宗龙,葛力源,杜坦,吴雅倩,宋一萌,刘承,马潞林. 肾透明细胞癌中核蛋白1对阿昔替尼耐药的作用及机制[J]. 北京大学学报(医学版), 2023, 55(5): 781-792. |
| [2] | 曹芳,钟明,刘从容. 宫体POLE突变型内膜样癌合并HPV感染相关性宫颈腺癌1例报道及文献回顾[J]. 北京大学学报(医学版), 2023, 55(2): 370-374. |
| [3] | 梁丽,李鑫,农琳,董颖,张继新,李东,李挺. 子宫内膜癌微卫星不稳定性分析: 微小微卫星变换的意义[J]. 北京大学学报(医学版), 2023, 55(2): 254-261. |
| [4] | 张波. 弥漫性神经内分泌细胞肿瘤病理学:共性与异质性[J]. 北京大学学报(医学版), 2023, 55(2): 210-216. |
| [5] | 柯杨,王敏敏,刘萌飞,刘芳芳,刘英,何忠虎. 肿瘤早期预警生物标志物的研究与思考[J]. 北京大学学报(医学版), 2022, 54(5): 810-813. |
| [6] | 左美妮,杜依青,于路平,戴翔,徐涛. 代谢综合征与肾透明细胞癌患者预后的相关性[J]. 北京大学学报(医学版), 2022, 54(4): 636-643. |
| [7] | 陈怀安,刘硕,李秀君,王哲,张潮,李凤岐,苗文隆. 炎症生物标志物对输尿管尿路上皮癌患者预后预测的临床价值[J]. 北京大学学报(医学版), 2021, 53(2): 302-307. |
| [8] | 杨飞龙,洪锴,赵国江,刘承,宋一萌,马潞林. 基于长链非编码RNA的生物信息学分析构建膀胱癌预后模型并确定预后生物标志物[J]. 北京大学学报(医学版), 2019, 51(4): 615-622. |
| [9] | 贺大林,徐珊,郭鹏. 外泌体在泌尿系肿瘤精准诊断中的应用[J]. 北京大学学报(医学版), 2017, 49(4): 561-564. |
| [10] | 刘瑾,熊耕砚,唐琦,方冬,李学松,周利群. 上尿路尿路上皮癌中RASSF1A基因启动子区域的甲基化状态及其临床意义[J]. 北京大学学报(医学版), 2016, 48(4): 571-578. |
|
||