北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (5): 907-919. doi: 10.19723/j.issn.1671-167X.2022.05.018
荆防颗粒中抑制新型冠状病毒蛋白酶3CLpro及PLpro的活性成分
- 北京大学药学院天然药物及仿生药物国家重点实验室,北京大学云南白药国际医学研究中心,北京 100191
Bioactive compounds of Jingfang Granules against SARS-CoV-2 virus proteases 3CLpro and PLpro
Zhan-peng SHANG,Yang YI,Rong YU,Jing-jing FAN,Yu-xi HUANG,Xue QIAO,Min YE*( )
)
- State Key Laboratory of Natural and Biomimetic Drugs, Peking University-Yunnan Baiyao International Medical Research Center, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China
摘要:
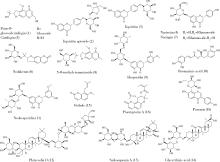
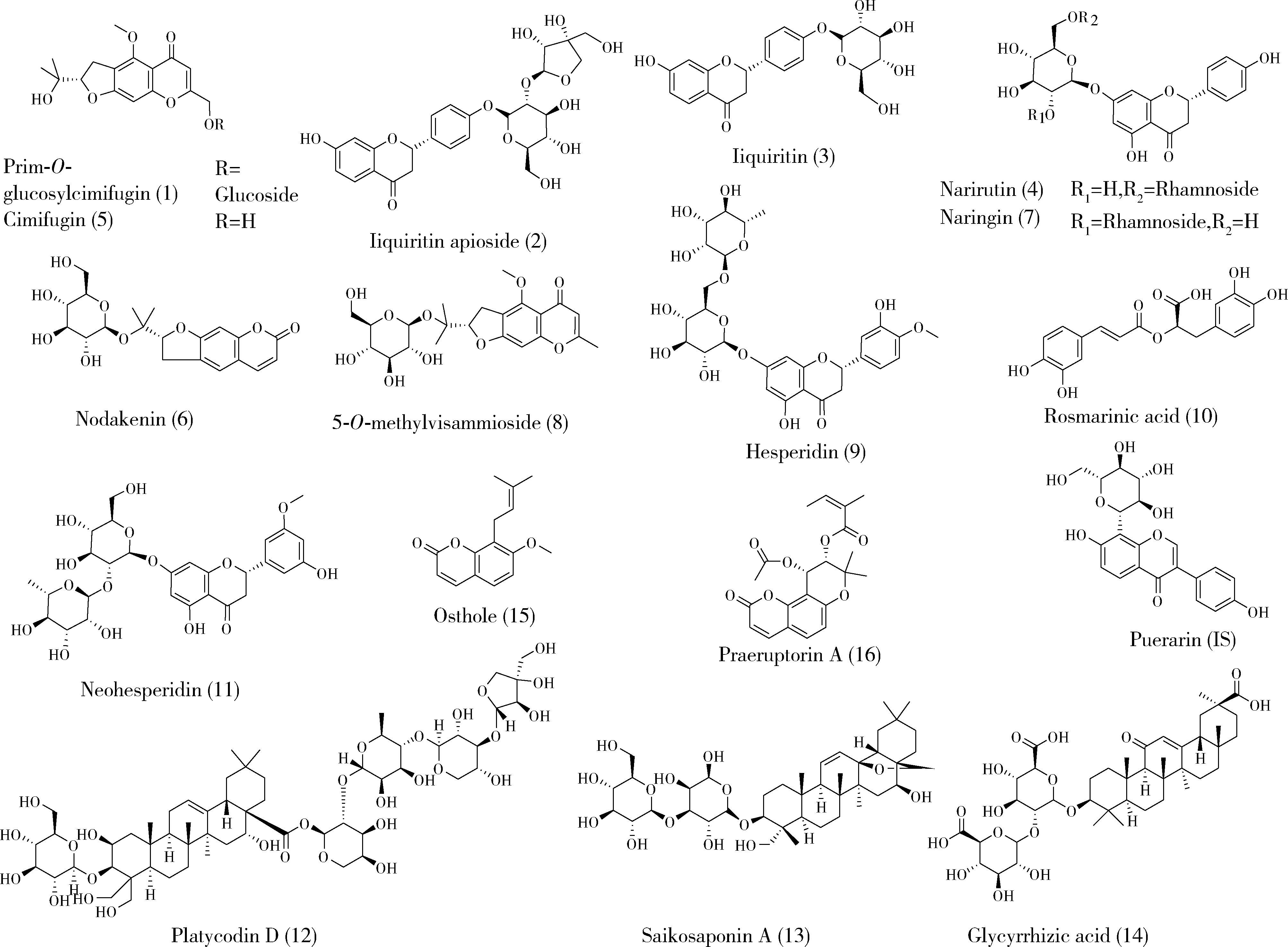
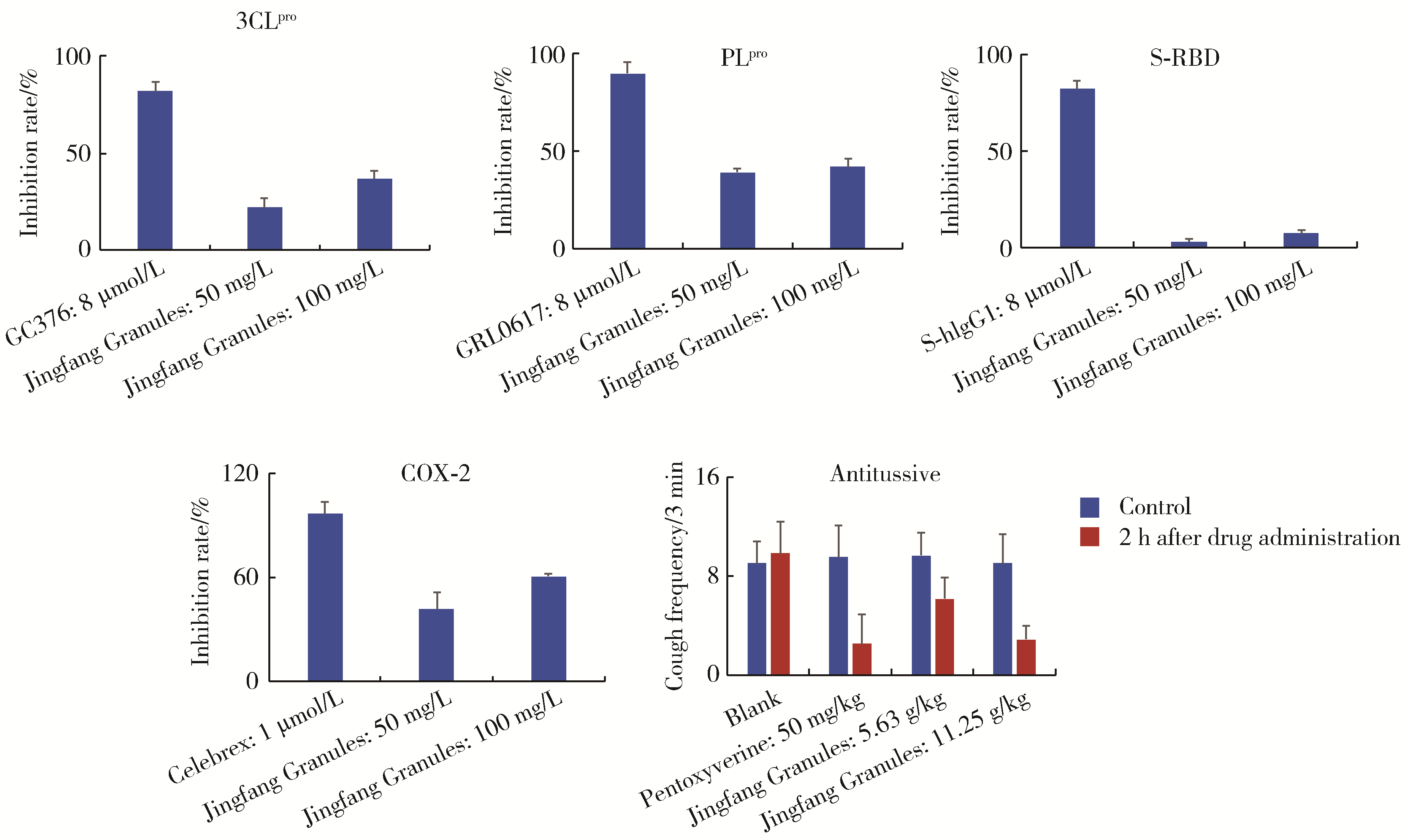
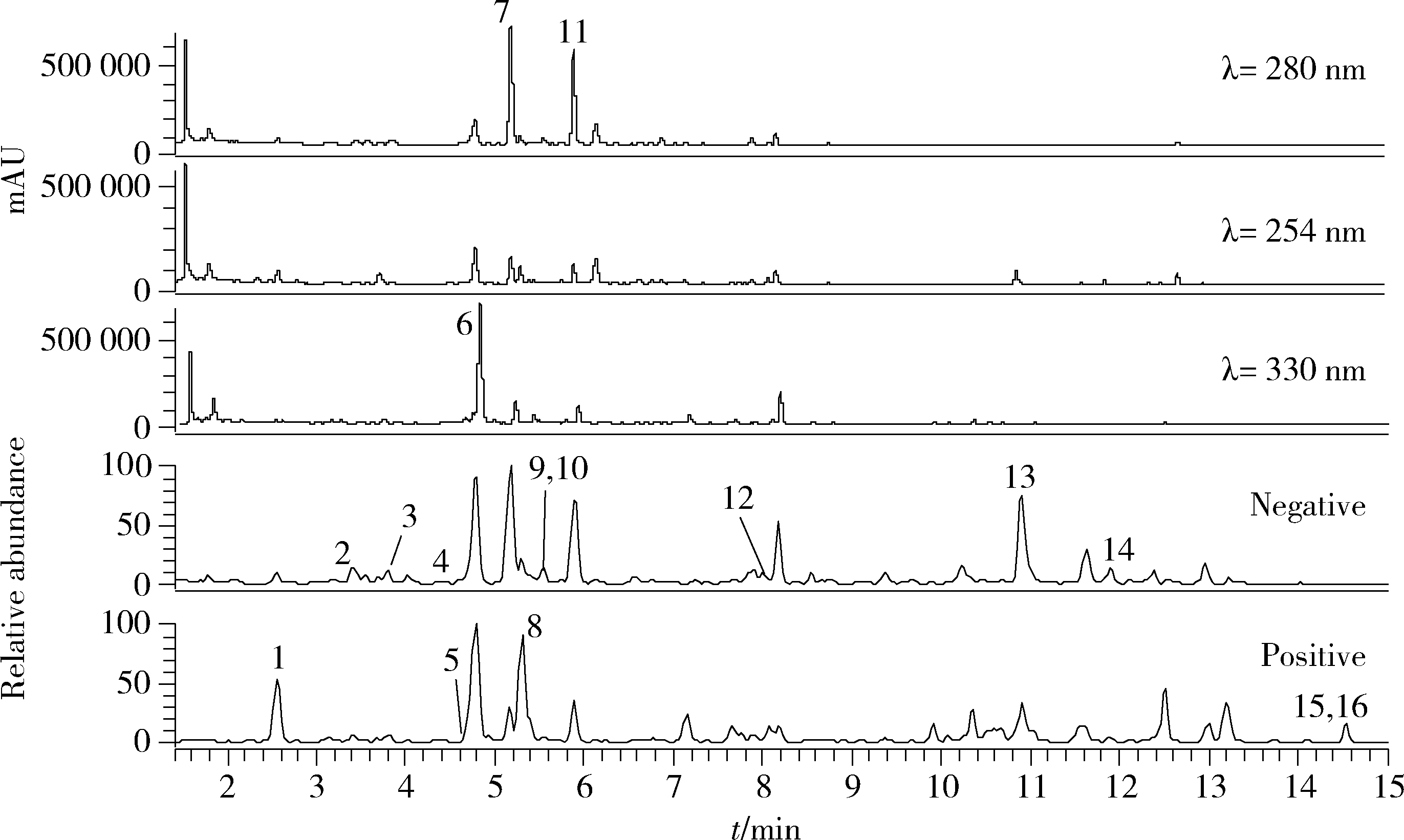
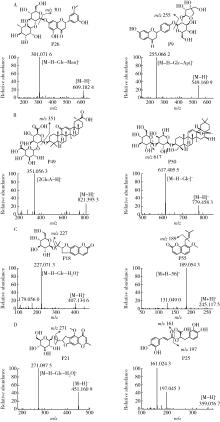
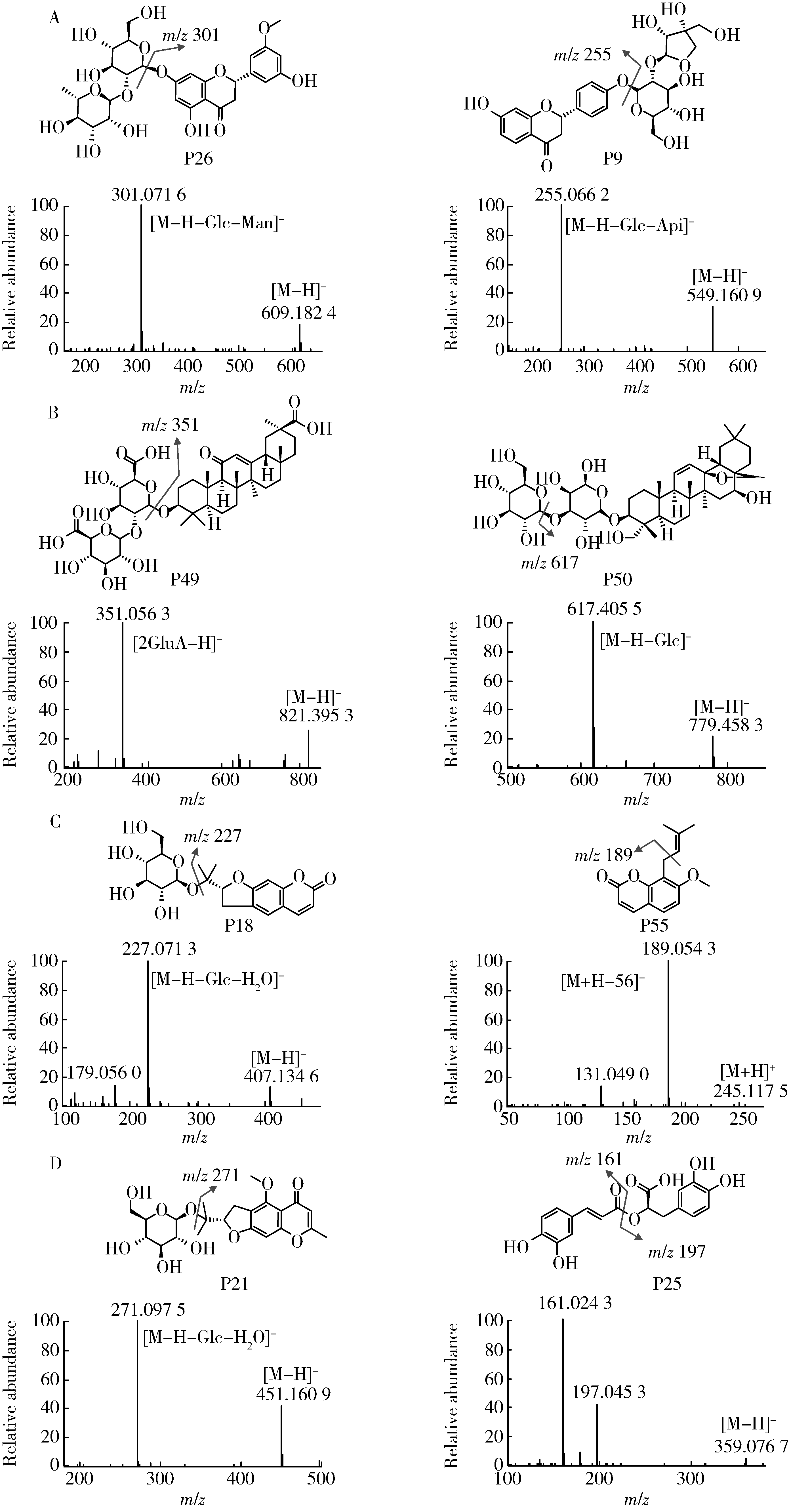
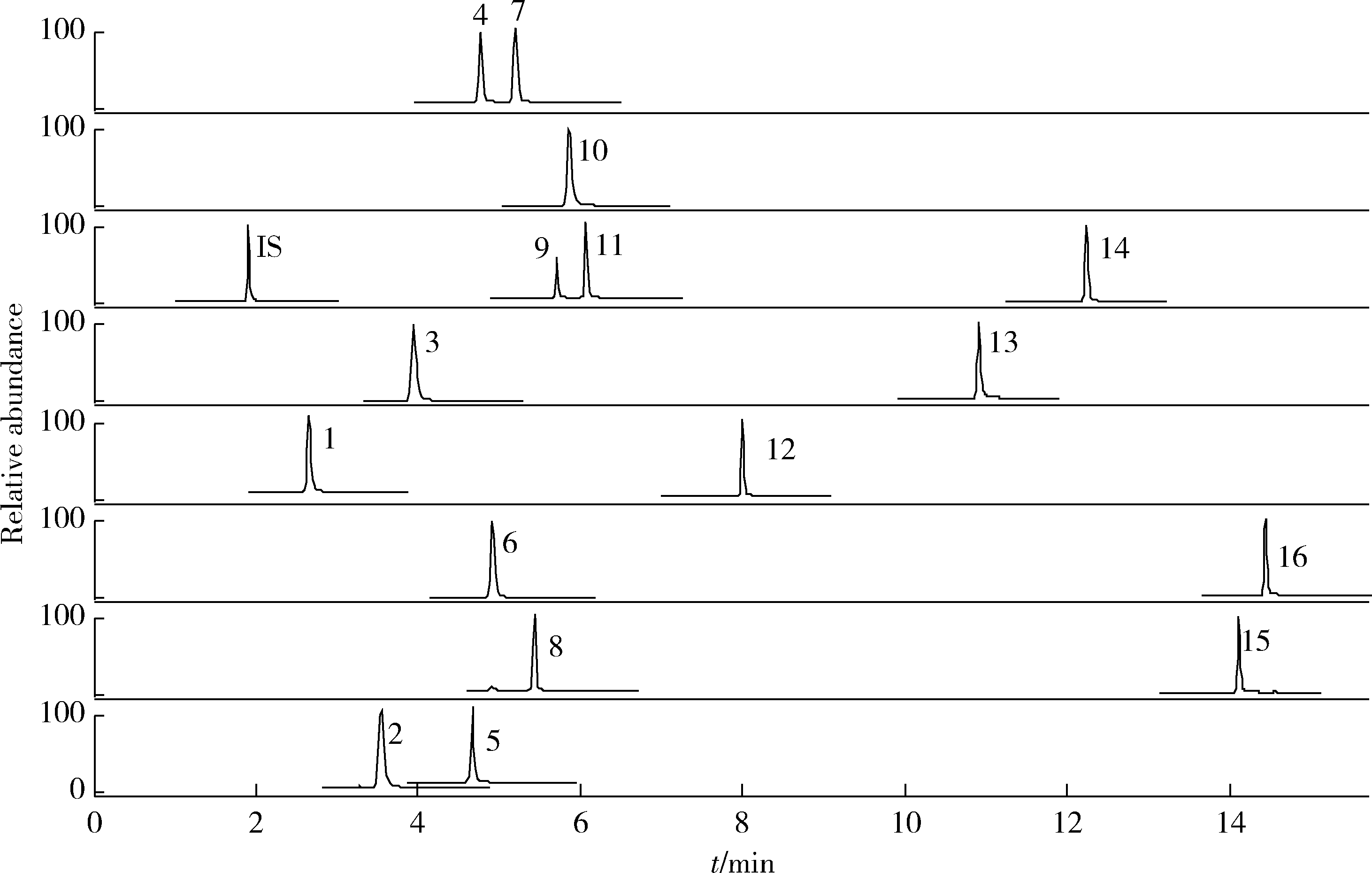
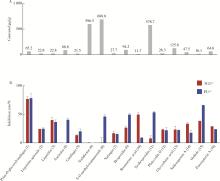
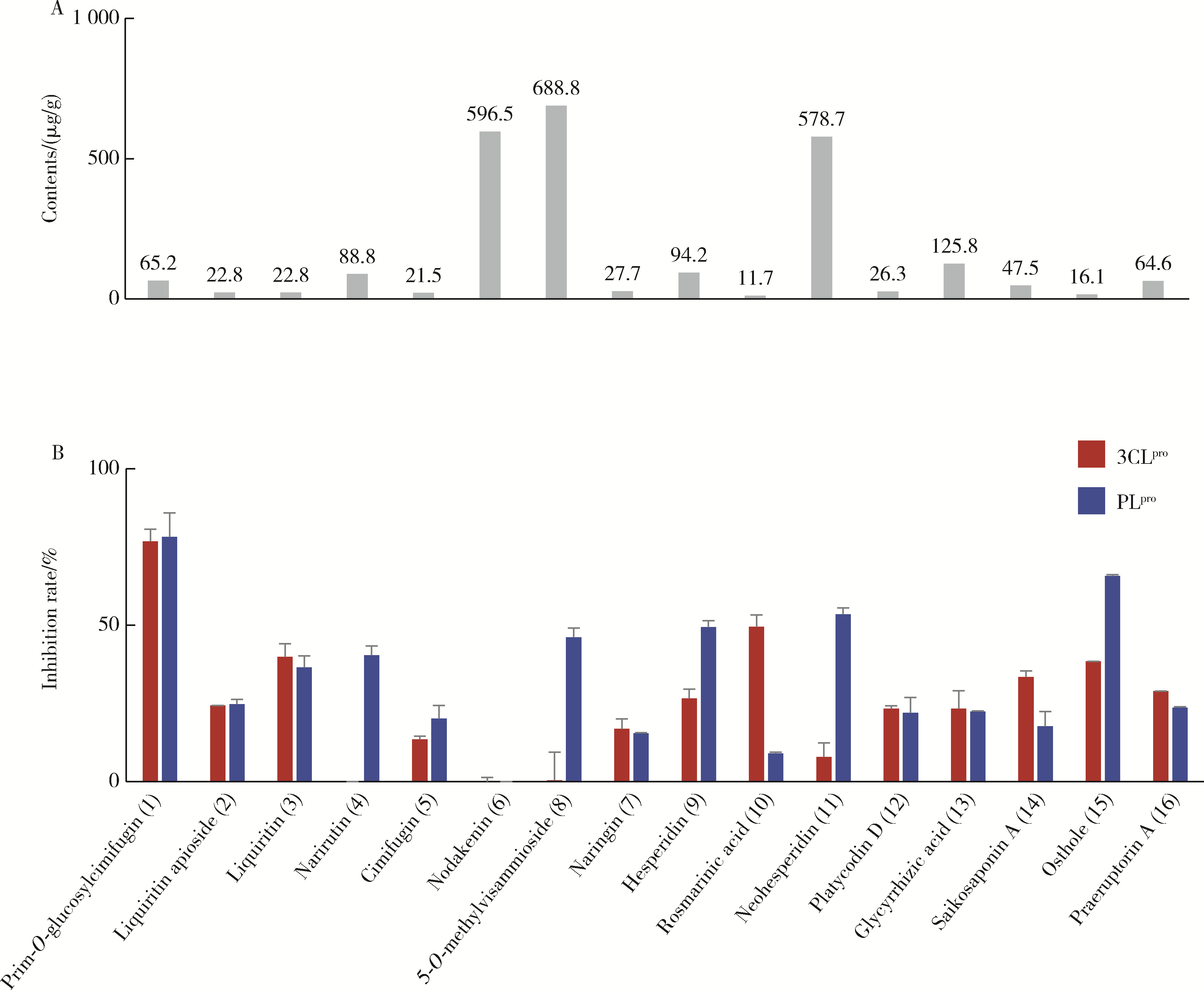
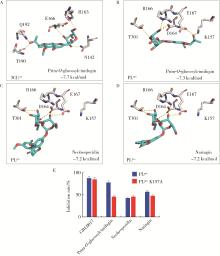
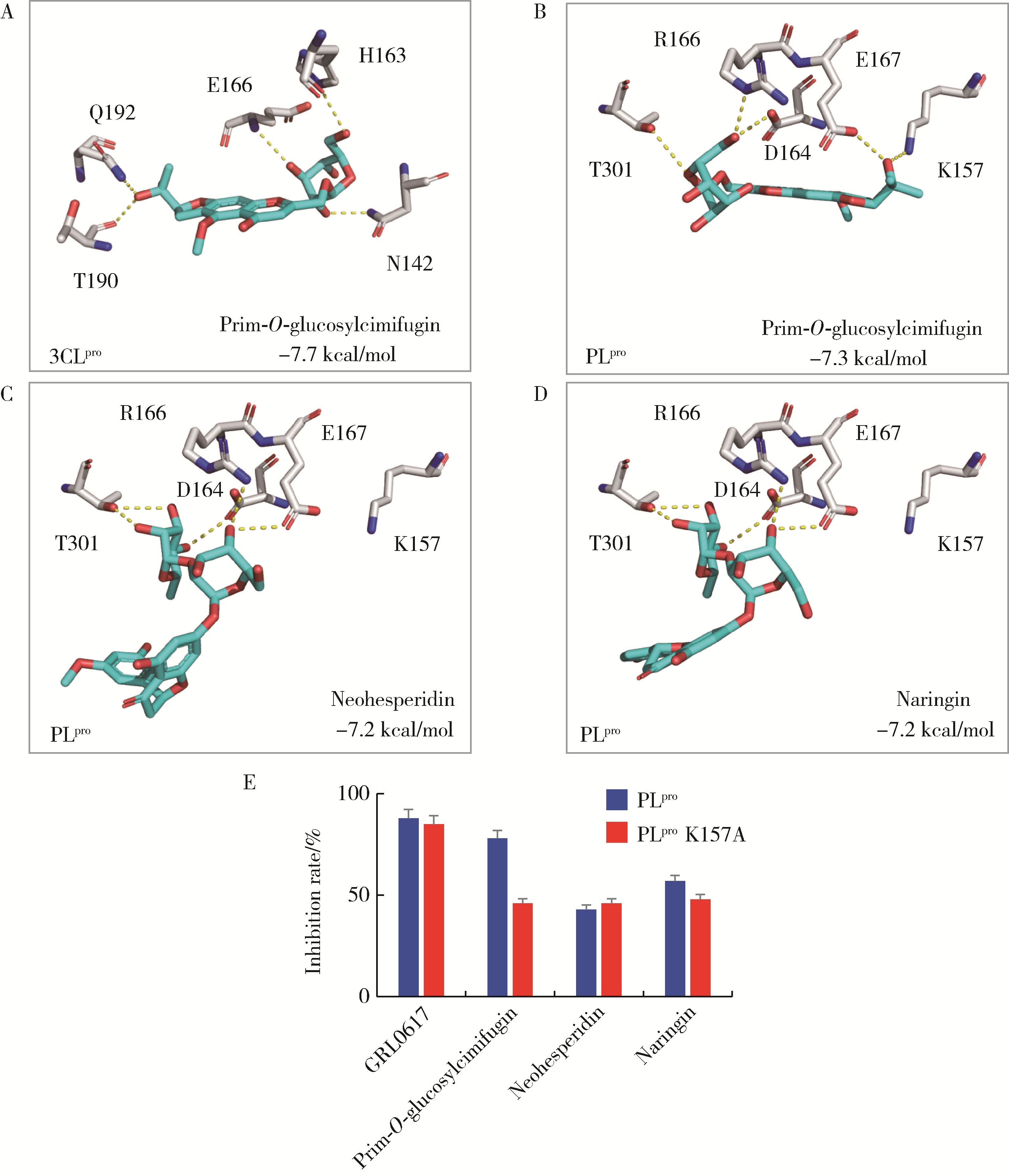
目的: 荆防颗粒是新型冠状病毒肺炎预防及轻症治疗的推荐用药,本文结合化学成分分析及活性检测,阐明其潜在的活性成分。方法: 采用酶学方法测定荆防颗粒提取物对新型冠状病毒3-chymotrypsin-like protease (3CLpro)、papain like protease (PLpro)、spike protein receptor-binding domain (S-RBD)及人cyclooxygenase-2 (COX-2)的抑制活性,利用氨水引咳小鼠模型测试其止咳作用;基于liquid chromatography-mass spectrometry(LC/MS)技术对荆防颗粒进行化学成分定性、定量分析,阐明其化学组成;采用酶学实验、分子对接、定点突变等方法测定荆防颗粒中抑制3CLpro、PLpro的主要活性成分并阐明可能的作用机制。结果: 荆防颗粒提取物对新型冠状病毒3CLpro、PLpro蛋白酶具有一定的抑制作用,且具有COX-2抑制活性及止咳药效。进一步在荆防颗粒鉴定了56个成分,其中16个成分经与对照品比对,准确鉴定其化学结构,并测定了其含量,总量为2 498.8 μg/g。16个成分中的主要成分升麻素苷对3CLpro和PLpro均具有显著的抑制活性,8 μmol/L的抑制率分别为76.8%和78.2%;新橙皮苷、柚皮苷对PLpro有抑制活性,8 μmol/L的抑制率分别为53.5%和46.1%。分子对接结果表明,升麻素苷可与3CLpro、PLpro活性口袋的氨基酸残基形成氢键,结合能分别为-7.7和-7.3 kcal/mol。定点突变结果表明,氨基酸残基K157是升麻素苷与PLpro相互作用的重要活性位点。结论: 荆防颗粒的主要成分升麻素苷、新橙皮苷、柚皮苷等具有抑制新型冠状病毒3CLpro及PLpro的活性,为荆防颗粒的临床合理使用提供了依据。
中图分类号:
- R932
| 1 |
Huang C , Wang Y , Li X , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020, 395 (10223): 497- 506.
doi: 10.1016/S0140-6736(20)30183-5 |
| 2 | World Health Organization. WHO coronavirus (COVID-19) dashboard[EB/OL]. (2022-04-29)[2022-05-01]. https://covid19.who.int. 2021. |
| 3 | Merck. Merck and ridgeback's molnupiravir, an oral COVID-19 antiviral medicine, receives first authorization in the world[EB/OL]. (2021-11-04)[2022-04-15]. https://www.merck.com/news/merck-and-ridgebacks-molnupiravir-an-oral-covid-19-antiviral-medicine-receives-first-authorization-in-the-world. 2021. |
| 4 | Pfizer. Pfizer to provide U.S. government with 10 million treatment courses of investigational oral antiviral candidate to help combat COVID-19[EB/OL]. (2021-11-18)[2022-04-18]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provide-us-government-10-million-treatment-courses.html. 2021. |
| 5 |
Dai W , Zhang B , Jiang X , et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease[J]. Science, 2020, 368 (6497): 1331- 1335.
doi: 10.1126/science.abb4489 |
| 6 |
Fu Z , Huang B , Tang J , et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery[J]. Nat Commun, 2021, 12 (1): 488.
doi: 10.1038/s41467-020-20718-8 |
| 7 |
付新, 刘阳, 王雪梅, 等. 麻杏石甘汤的研究进展[J]. 中医药信息, 2017, 34 (2): 126- 128.
doi: 10.3969/j.issn.1002-2406.2017.02.037 |
| 8 |
赵琰, 胡杰, 张贵民, 等. 荆防败毒散的源流与应用[J]. 环球中医药, 2020, 13 (11): 1996- 2002.
doi: 10.3969/j.issn.1674-1749.2020.11.042 |
| 9 | 冯芹, 张贵民. 荆防败毒散治疗急性呼吸道感染的临床应用以及作用机制的探讨[J]. 中药与临床, 2020, 11 (3): 28- 32. |
| 10 | 邹胜. 荆防败毒散治疗急性病毒性上呼吸道感染[J]. 山西中医, 2010, 26 (3): 11- 12. |
| 11 |
殷健操, 周荣, 黄仁礼. 从"热入血室"论治新型冠状病毒肺炎产妇1例[J]. 光明中医, 2020, 35 (16): 2560- 2562.
doi: 10.3969/j.issn.1003-8914.2020.16.047 |
| 12 | 张奎, 陈红英, 马瑜. 荆防败毒散药效学研究[J]. 河南中医, 2009, 29 (6): 601- 602. |
| 13 | Song Y , Jing W , Yan R , et al. Research progress of the studies on the roots of Peucedanum praeruptorum dunn (Peucedani Radix)[J]. Pak J Pharm Sci, 2015, 28 (1): 71- 81. |
| 14 | Xi J , Xiang S , Zhang H , et al. Clinical observation of arbidol combined with diammonium glycyrrhizinate in the treatment of COVID-19[J]. Chin J Hosp Phar, 2020, 40 (12): 1287- 1290. |
| 15 |
Shi R , Xu JW , Xiao ZT , et al. Naringin and naringenin relax rat tracheal smooth by regulating BKCa activation[J]. J Med Food, 2019, 22 (9): 963- 970.
doi: 10.1089/jmf.2018.4364 |
| 16 | 刘雯, 李峰, 孙春亮, 等. HPLC同时测定荆防颗粒中6种成分[J]. 中国实验方剂学杂志, 2016, 22 (17): 55- 58. |
| 17 | 冯雪, 高玉乔, 范琼瑛, 等. 采用LC-ESI/MS方法同时测定荆防败毒口服液中4个有效成分含量[J]. 药物分析杂志, 2017, 37 (8): 1489- 1496. |
| 18 | 梁红宝, 姜宇, 袁晓梅, 等. 基于GC-MS和UPLC-Q-Exactive MS技术的荆防颗粒化学成分研究[J]. 中草药, 2022, 53 (6): 1697- 1708. |
| 19 |
Yi Y , Li J , Lai X , et al. Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection[J]. J Adv Res, 2022, 36, 201- 210.
doi: 10.1016/j.jare.2021.11.012 |
| 20 |
Shang Z , Xu L , Kuang Y , et al. Simultaneous determination of 35 constituents and elucidation of effective constituents in a multi-herb Chinese medicine formula Xiaoer-Feire-Kechuan[J]. J Pharm Anal, 2021, 11 (6): 717- 725.
doi: 10.1016/j.jpha.2021.01.003 |
| 21 |
Kuang Y , Li B , Fan J , et al. Antitussive and expectorant activities of licorice and its major compounds[J]. Bioorg Med Chem, 2018, 26 (1): 278- 284.
doi: 10.1016/j.bmc.2017.11.046 |
| 22 |
Shang ZP , Xu LL , Xiao Y , et al. A global profiling strategy using comprehensive two-dimensional liquid chromatography coupled with dual-mass spectrometry platforms: Chemical analysis of a multi-herb Chinese medicine formula as a case study[J]. J Chromatogr A, 2021, 1642, 462021.
doi: 10.1016/j.chroma.2021.462021 |
| 23 |
Song W , Qiao X , Chen K , et al. Biosynthesis-based quantitative analysis of 151 secondary metabolites of licorice to differentiate medicinal Glycyrrhiza species and their hybrids[J]. Anal Chem, 2017, 89 (5): 3146- 3153.
doi: 10.1021/acs.analchem.6b04919 |
| 24 |
Wang S , Qian Y , Sun M , et al. Holistic quality evaluation of Saposhnikoviae Radix (Saposhnikovia divaricata) by reversed-phase ultra-high performance liquid chromatography and hydrophi-lic interaction chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry-based untargeted metabolomics[J]. Arab J Chem, 2020, 13 (12): 8835- 8847.
doi: 10.1016/j.arabjc.2020.10.013 |
| 25 | Bai Y , Zheng Y , Pang W , et al. Identification and comparison of constituents of Aurantii Fructus and Aurantii Fructus Immaturus by UFLC-DAD-Triple TOF-MS/MS[J]. Molecules, 2018, 23 (4): 1- 15. |
| 26 |
Chu S , Chen L , Xie H , et al. Comparative analysis and chemical profiling of different forms of Peucedani Radix[J]. J Pharmaceut Biomed Anal, 2020, 189, 113410.
doi: 10.1016/j.jpba.2020.113410 |
| 27 |
Wan M , Zhang Y , Yang Y , et al. Analysis of the chemical composition of Angelicae Pubescentis Radix by ultra-performance liquid chromatography and quadrupole time-of-flight tandem mass spectrometry[J]. J Chin Pharm Sci, 2019, 28 (3): 145- 159.
doi: 10.5246/JCPS.2019.03.014 |
| 28 |
Huang W , Zhou H , Yuan M , et al. Comprehensive characterization of the chemical constituents in Platycodon grandiflorum by an integrated liquid chromatography-mass spectrometry strategy[J]. J Chromatogr A, 2021, 1654, 462477.
doi: 10.1016/j.chroma.2021.462477 |
| 29 | 郭敏娜, 刘素香, 赵艳敏, 等. 基于HPLC-Q-TOF-MS技术的柴胡化学成分分析[J]. 中草药, 2016, 12 (47): 2044- 2052. |
| 30 | 郑单单, 魏文峰, 霍金海, 等. 基于UPLC-Q-TOF-MS技术的芪风固表颗粒血清药物化学研究[J]. 中草药, 2021, 52 (3): 643- 652. |
| 31 | 魏飞亭, 程昊, 乔日发, 等. UPLC-Q-TOF/MS鉴定大鼠灌服枳壳提取物后的入血成分及其代谢产物[J]. 中国实验方剂学杂志, 2020, 26 (21): 161- 172. |
| [1] | 刘鑫,石雪迎,李军. 新型冠状病毒感染相关缺血性结肠炎1例[J]. 北京大学学报(医学版), 2024, 56(2): 362-365. |
| [2] | 朱金荣,赵亚娜,黄巍,赵微微,王悦,王松,苏春燕. 感染新型冠状病毒的血液透析患者的临床特征[J]. 北京大学学报(医学版), 2024, 56(2): 267-272. |
| [3] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [4] | 赖金惠,王起,姬家祥,王明瑞,唐鑫伟,许克新,徐涛,胡浩. 新型冠状病毒肺炎疫情期间延迟拔除输尿管支架对泌尿系结石术后患者生活质量和心理状态的影响[J]. 北京大学学报(医学版), 2023, 55(5): 857-864. |
| [5] | 康志宇,王磊磊,韩永正,郭向阳. 北京冬季奥林匹克运动会运动员手术的麻醉管理[J]. 北京大学学报(医学版), 2022, 54(4): 770-773. |
| [6] | 陈明隆,刘笑晗,郭静. 新型冠状病毒肺炎疫情下儿童父母社会支持与养育倦怠的关系[J]. 北京大学学报(医学版), 2022, 54(3): 520-525. |
| [7] | 李秋钰,程秦,赵志伶,代妮妮,曾琳,朱兰,郭炜,李超,王军红,李姝,葛庆岗,沈宁. 肾移植术后感染新型冠状病毒1例[J]. 北京大学学报(医学版), 2020, 52(4): 780-784. |
| [8] | 杨航,杨林承,张瑞涛,凌云鹏,葛庆岗. 合并高血压、冠心病、糖尿病的新型冠状病毒肺炎患者发生病死的危险因素分析[J]. 北京大学学报(医学版), 2020, 52(3): 420-424. |
| [9] | 陈美恋,高燕,郭维,左力,王天兵. 新型冠状病毒肺炎患者床旁血液净化治疗的感染防控[J]. 北京大学学报(医学版), 2020, 52(3): 414-419. |
| [10] | 邢燕,张娟,韩彤妍,李在玲,李蕊,童笑梅. 新型冠状病毒感染肺炎疫情下综合医院儿科防控方式探索[J]. 北京大学学报(医学版), 2020, 52(3): 410-413. |
| [11] | 屠鹏飞, 郭洪祝, 果德安. 中药与天然药物活性成分研究及新药的发现[J]. 北京大学学报(医学版), 2002, 34(5): 513-518. |
|
||