北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (1): 124-132. doi: 10.19723/j.issn.1671-167X.2023.01.019
初诊IgA肾病患者的肠道菌群及其与疾病进展因素的相关分析
- 北京大学第三医院肾内科,北京 100191
Changes of gut microflora in newly diagnosed IgA nephropathy patients and its correlation with clinical risk factors
- Department of Nephrology, Peking University Third Hospital, Beijing 100191, China
摘要:
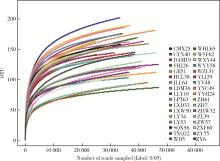
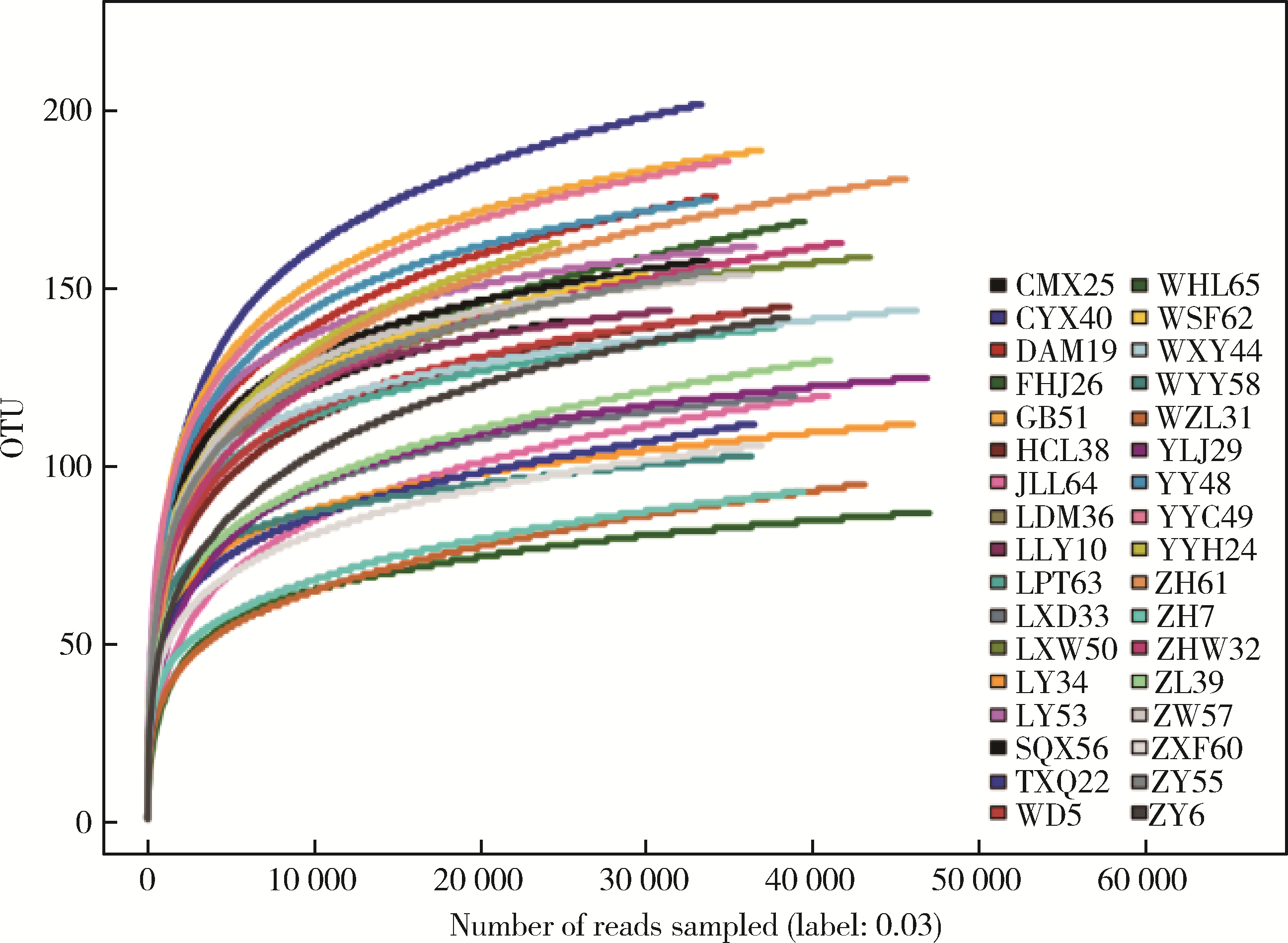
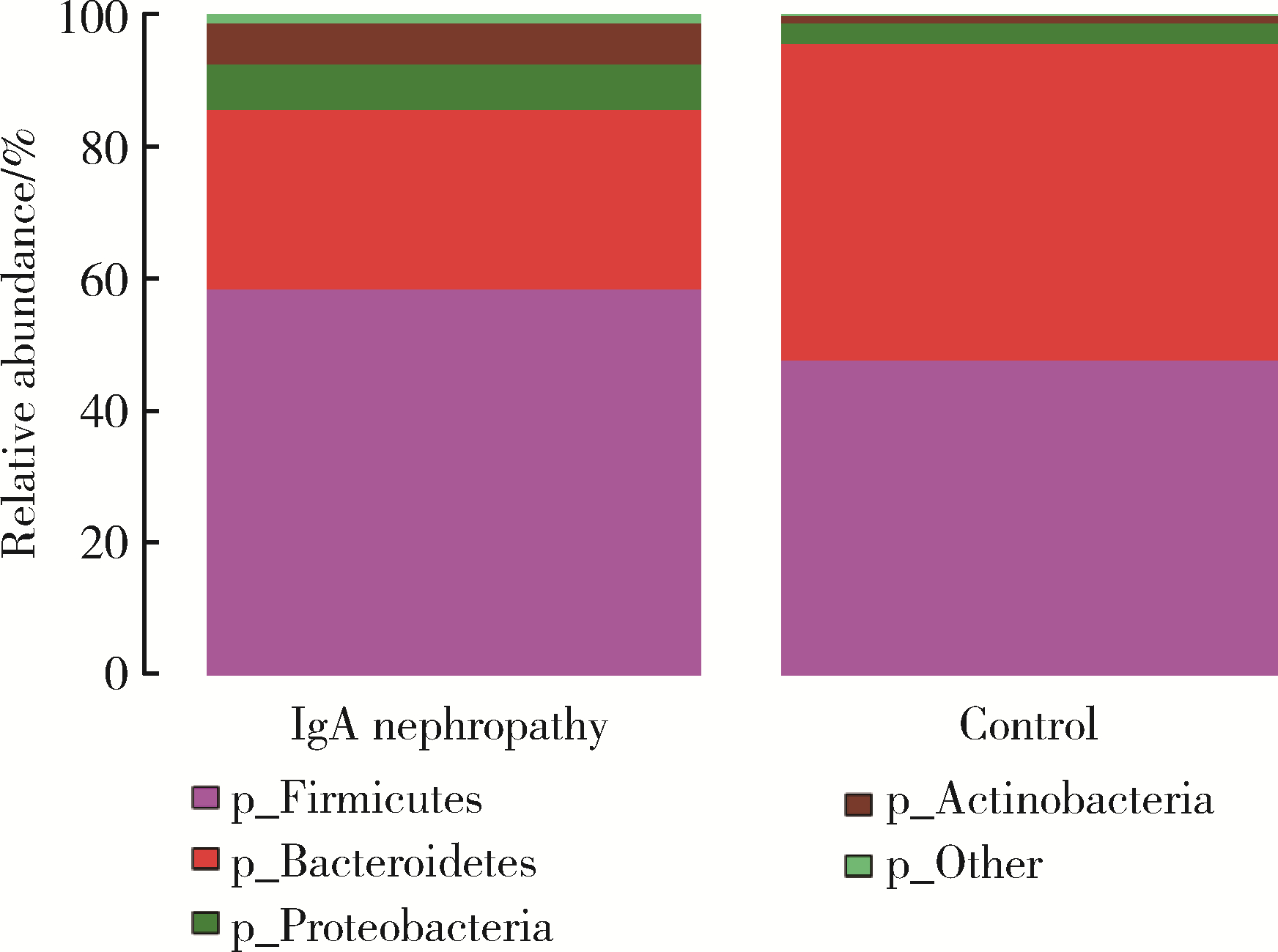
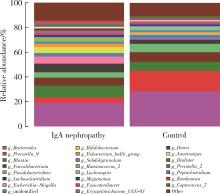
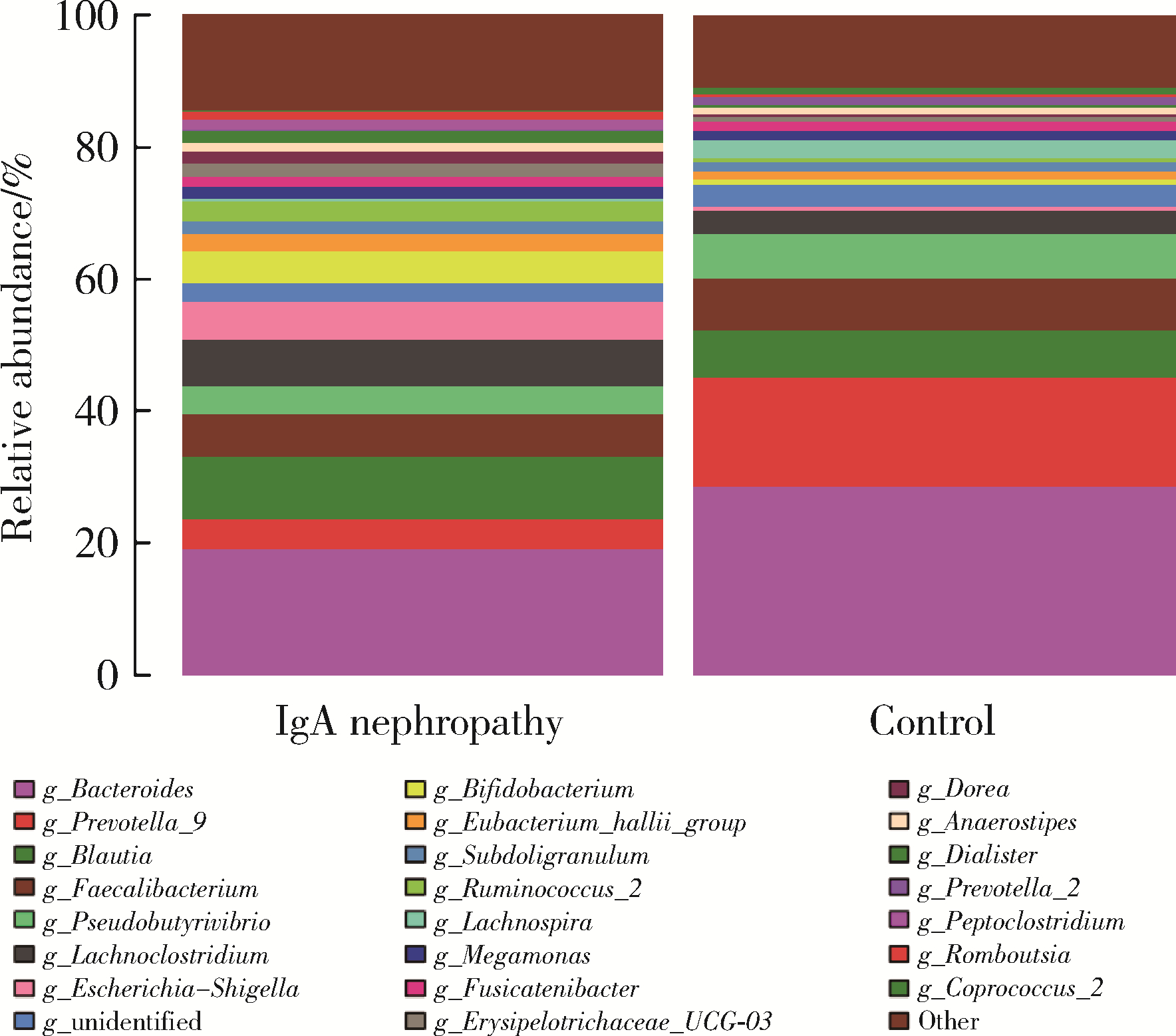
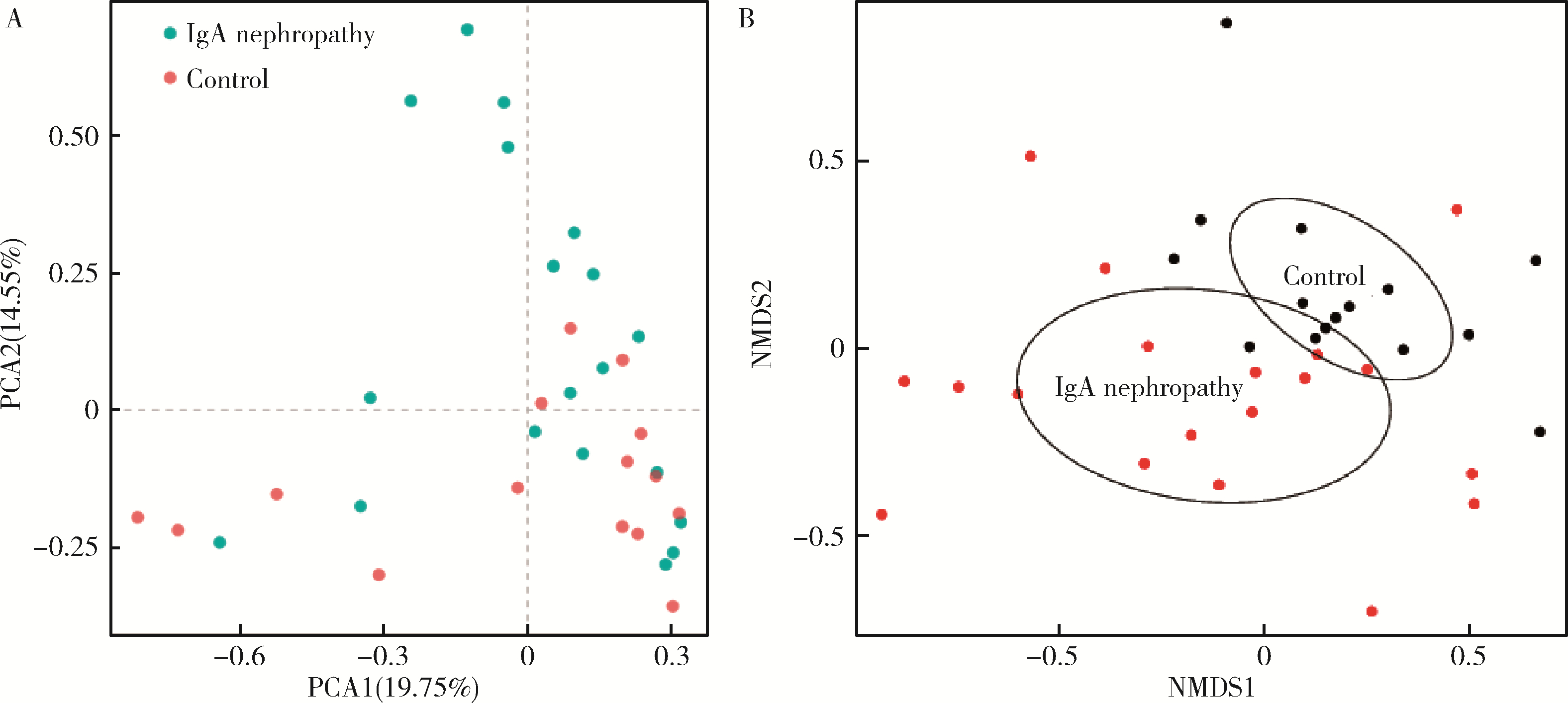
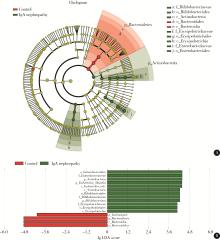
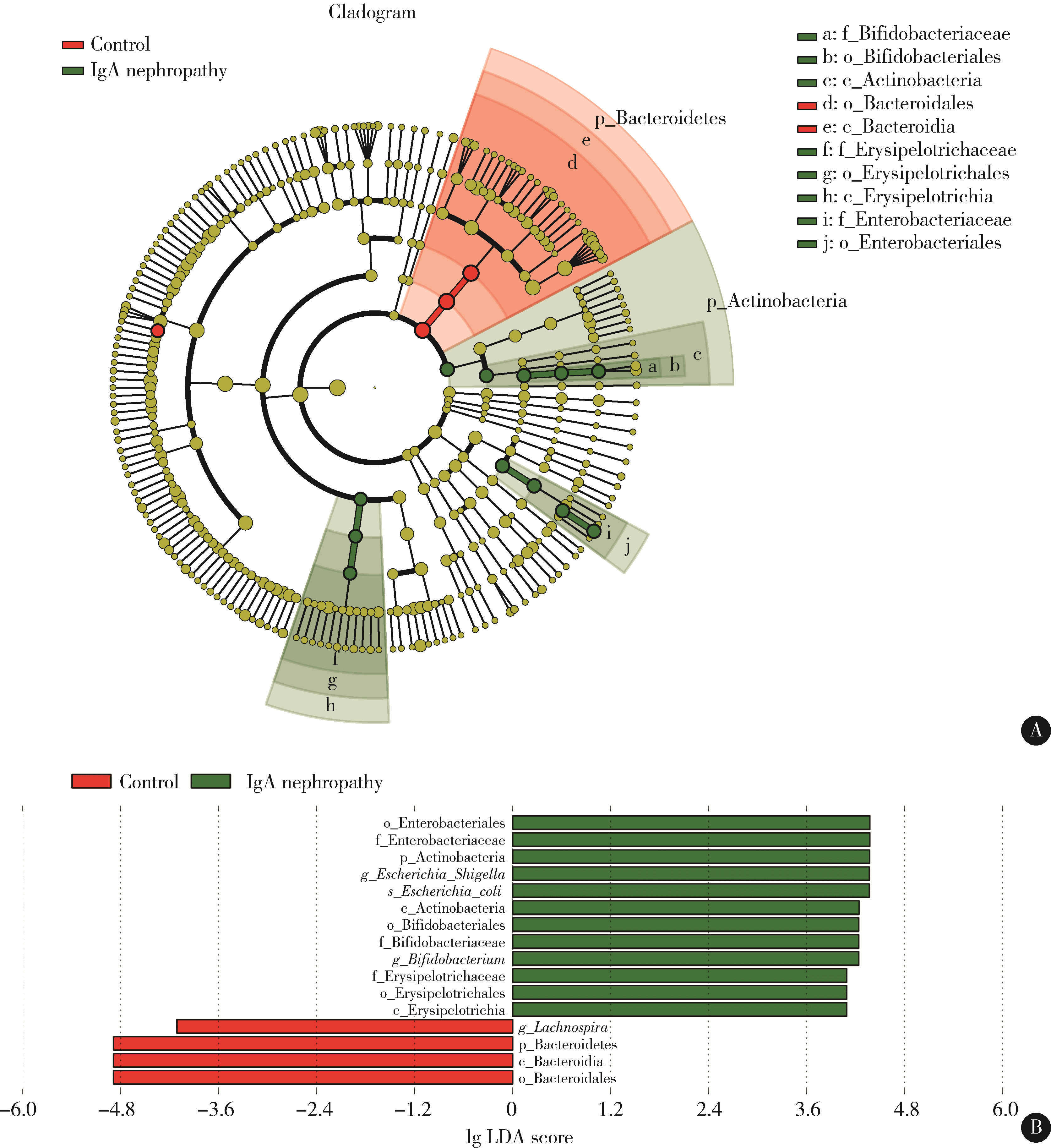
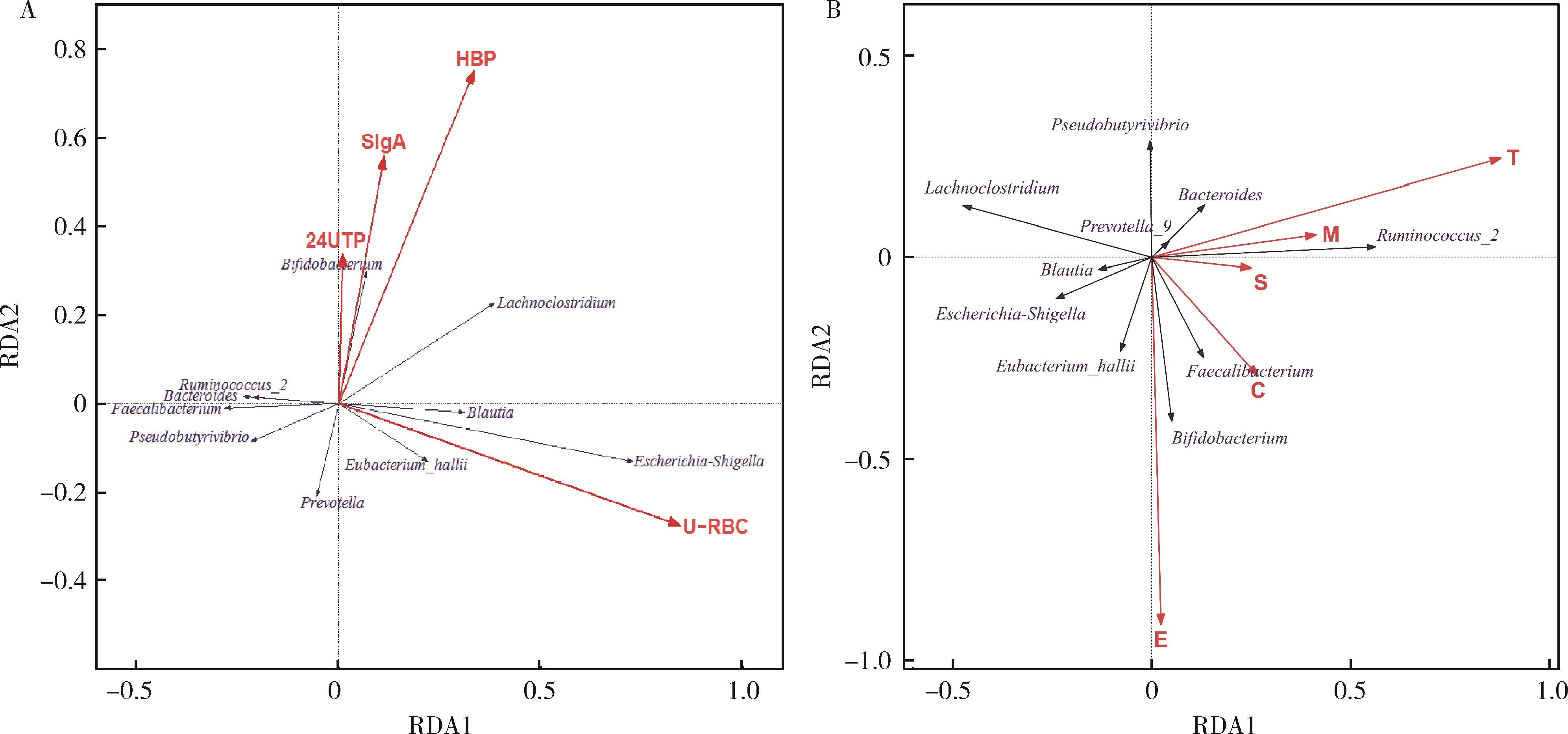
目的: 观察慢性肾脏病(chronic kidney disease,CKD)1~2期的初诊IgA肾病患者肠道微生物群的特征,进一步探索IgA肾病疾病进展因素与肠道微生物之间的关系。方法: 收集19例CKD 1~2期的初诊IgA肾病患者和15例年龄、性别相匹配的健康对照组的新鲜粪便样本,提取粪便细菌DNA,针对V3-V4区域进行16S核糖体RNA(16S ribosomal RNA,16S rRNA)高通量测序分析肠道菌群的组成,应用Illumina Miseq平台对粪便菌群测序结果进行分析。收集初诊IgA肾病患者的疾病进展因素,研究肠道菌群与IgA肾病疾病进展因素之间的相关性。结果: (1) 与健康对照组相比,在门水平上,拟杆菌门(Bacteroidetes)的丰度显著降低(P=0.046),放线菌门(Actinobacteria)的丰度显著升高(P=0.001)。在属水平上,埃希菌-志贺菌属(Escherichia-Shigella)、双歧杆菌属(Bifidobacterium)、Dorea等菌属的丰度显著升高(P < 0.05),毛螺菌属(Lachnospira)、粪球菌属_2(Coprococcus_2)、萨特氏菌属(Sutterella)等菌属的丰度显著降低(P < 0.05)。(2)初诊IgA肾病患者与健康对照组之间肠道菌群的丰度比较差异无统计学意义(P>0.05),但两组肠道菌群的组成存在差异。LEfSe分析结果显示,初诊IgA肾病患者和健康对照组比较有16个差异菌,其中,初诊IgA肾病患者肠杆菌目(Enterobacteriales)、放线菌门、埃希菌-志贺菌属等的丰度升高,健康对照组拟杆菌门、毛螺菌属的丰度升高。(3)冗余分析(redundancy analysis,RDA)的结果显示,初诊IgA肾病患者粪便中双歧杆菌属与血清IgA水平、24 h尿蛋白定量和合并高血压呈正相关,Lachnoclostridium菌属与合并高血压呈正相关,埃希菌-志贺菌属与尿红细胞数量呈正相关,双歧杆菌属与毛细血管内增殖呈正相关,粪杆菌属与细胞/纤维细胞性新月体呈正相关,瘤胃球菌属_2(Ruminococcus_2)与系膜细胞增殖、肾小球节段硬化、肾小管萎缩/间质纤维化呈正相关。结论: 初诊CKD 1~2期IgA肾病患者的肠道菌群与健康对照相比存在差异,肠道的某些菌属与IgA肾病疾病进展因素呈相关性,需要进一步的研究来了解这些菌属在IgA肾病中的潜在作用。
中图分类号:
- R692.3
| 1 |
Schena FP , Nistor I . Epidemiology of IgA nephropathy: A global perspective[J]. Semin Nephrol, 2018, 38 (5): 435- 442.
doi: 10.1016/j.semnephrol.2018.05.013 |
| 2 |
Wang M , Lv J , Zhang X , et al. Secondary IgA nephropathy shares the same immune features with primary IgA nephropathy[J]. Kidney Int Rep, 2020, 5 (2): 165- 172.
doi: 10.1016/j.ekir.2019.10.012 |
| 3 |
Hu X , Du J , Xie Y , et al. Fecal microbiota characteristics of Chinese patients with primary IgA nephropathy: A cross-sectional study[J]. BMC Nephrol, 2020, 21 (1): 97.
doi: 10.1186/s12882-020-01741-9 |
| 4 |
De Angelis M , Montemurno E , Piccolo M , et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN)[J]. PLoS One, 2014, 9 (6): e99006.
doi: 10.1371/journal.pone.0099006 |
| 5 |
Hobby GP , Karaduta O , Dusio GF , et al. Chronic kidney disease and the gut microbiome[J]. Am J Physiol Renal Physiol, 2019, 316 (6): F1211- F1217.
doi: 10.1152/ajprenal.00298.2018 |
| 6 |
Munyaka PM , Eissa N , Bernstein CN , et al. Antepartum antibio-tic treatment increases offspring susceptibility to experimental colitis: A role of the gut microbiota[J]. PLoS One, 2015, 10 (11): e0142536.
doi: 10.1371/journal.pone.0142536 |
| 7 |
Edgar RC . UPARSE: highly accurate OTU sequences from microbial amplicon reads[J]. Nat Methods, 2013, 10 (10): 996- 998.
doi: 10.1038/nmeth.2604 |
| 8 | Cole JR , Wang Q , Cardenas E , et al. The ribosomal database project: Improved alignments and new tools for rRNA analysis[J]. Nucleic Acids Res, 2009, 37 (Suppl 1): D141- D145. |
| 9 |
Segata N , Izard J , Waldron L , et al. Metagenomic biomarker discovery and explanation[J]. Genome Biol, 2011, 12 (6): R60.
doi: 10.1186/gb-2011-12-6-r60 |
| 10 |
Mitchell RJ , Hewison RL , Fielding DA , et al. Decline in atmospheric sulphur deposition and changes in climate are the major drivers of long-term change in grassland plant communities in Scotland[J]. Environ Pollut, 2018, 235, 956- 964.
doi: 10.1016/j.envpol.2017.12.086 |
| 11 | Li H , Li T , Beasley DE , et al. Diet diversity is associated with beta but not alpha diversity of pika gut microbiota[J]. Front Microbiol, 2016, 7, 1169. |
| 12 |
Suzuki H , Kiryluk K , Novak J , et al. The pathophysiology of IgA nephropathy[J]. J Am Soc Nephrol, 2011, 22 (10): 1795- 1803.
doi: 10.1681/ASN.2011050464 |
| 13 |
Nakajima A , Vogelzang A , Maruya M , et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria[J]. J Exp Med, 2018, 215 (8): 2019- 2034.
doi: 10.1084/jem.20180427 |
| 14 |
Floege J , Feehally J . The mucosa-kidney axis in IgA nephropathy[J]. Nat Rev Nephrol, 2016, 12 (3): 147- 156.
doi: 10.1038/nrneph.2015.208 |
| 15 |
Olive C , Allen AC , Harper SJ , et al. Expression of the mucosal T cell receptor V region repertoire in patients with IgA nephropathy[J]. Kidney Int, 1997, 52 (4): 1047- 1053.
doi: 10.1038/ki.1997.427 |
| 16 |
McCarthy DD , Kujawa J , Wilson C , et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy[J]. J Clin Invest, 2011, 121 (10): 3991- 4002.
doi: 10.1172/JCI45563 |
| 17 |
Kiryluk K , Li Y , Scolari F , et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens[J]. Nat Genet, 2014, 46 (11): 1187- 1196.
doi: 10.1038/ng.3118 |
| 18 |
Grosserichter-Wagener C , Radjabzadeh D , van der Weide H , et al. Differences in systemic IgA reactivity and circulating Th subsets in healthy volunteers with specific microbiota enterotypes[J]. Front Immunol, 2019, 10, 341.
doi: 10.3389/fimmu.2019.00341 |
| 19 |
Barko PC , McMichael MA , Swanson KS , et al. The gastrointestinal microbiome: A review[J]. J Vet Intern Med, 2018, 32 (1): 9- 25.
doi: 10.1111/jvim.14875 |
| 20 |
Gomaa EZ . Human gut microbiota/microbiome in health and diseases: A review[J]. Antonie Van Leeuwenhoek, 2020, 113 (12): 2019- 2040.
doi: 10.1007/s10482-020-01474-7 |
| 21 |
包文晗, 王悦. 慢性肾脏病患者肠道菌群的变化及影响研究[J]. 中国全科医学, 2018, 21 (24): 2927- 2931.
doi: 10.3969/j.issn.1007-9572.2018.00.168 |
| 22 |
Gibiino G , Lopetuso LR , Scaldaferri F , et al. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals[J]. Dig Liver Dis, 2018, 50 (7): 635- 639.
doi: 10.1016/j.dld.2018.03.016 |
| 23 |
Lo Presti A , Zorzi F , Del Chierico F , et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease[J]. Front Microbiol, 2019, 10, 1655.
doi: 10.3389/fmicb.2019.01655 |
| 24 |
Amabebe E , Robert FO , Agbalalah T , et al. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism[J]. Br J Nutr, 2020, 123 (10): 1127- 1137.
doi: 10.1017/S0007114520000380 |
| 25 |
Coppo R . The gut-kidney axis in IgA nephropathy: Role of microbiota and diet on genetic predisposition[J]. Pediatr Nephrol, 2018, 33 (1): 53- 61.
doi: 10.1007/s00467-017-3652-1 |
| 26 | Liong MT . Beneficial microorganisms in medical and health applications[M]. Switzerland: Springer International Publishing, 2015. |
| [1] | 张家赫,史佳琪,陈章健,贾光. 基于人消化道微生态体外模拟系统观察纳米二氧化钛对肠道菌群的影响[J]. 北京大学学报(医学版), 2022, 54(3): 468-476. |
| [2] | 王子靖,李在玲. 有幽门螺杆菌感染家族史儿童胃部菌群的特点[J]. 北京大学学报(医学版), 2021, 53(6): 1115-1121. |
| [3] | 张梅香,史文芝,刘建新,王春键,李燕,王蔚,江滨. MLL-AF6融合基因阳性急性髓系白血病的临床特征及预后[J]. 北京大学学报(医学版), 2021, 53(5): 915-920. |
| [4] | 颜勇卿, 张培训 , 王天兵 , 陈建海, 姜保国. 手术治疗的桡骨远端骨折多中心社会学与临床特点分析[J]. 北京大学学报(医学版), 2014, 46(2): 254-257. |
|
||