北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (3): 578-583. doi: 10.19723/j.issn.1671-167X.2025.03.023
高海拔地区结直肠良恶性肿瘤患者肠道菌群差异及其与低海拔地区正常人群的比较
- 1. 西藏大学医学院, 西藏自治区人民医院消化内科, 拉萨 850000
2. 北京大学第三医院消化科, 北京 100191
Changes of intestinal microflora in patients with colorectal benign and malignant tumors in high altitude area and comparison with the normal population in low altitude area
Dan HAN1, Yangjin CIREN1, Qiuhong LI2, Jun LI2,*( )
)
- 1. Department of Gastroenterology, Tibet University School of Medicine, People's Hospital of Tibet Autonomous Region, Lasa 850000, China
2. Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China
摘要:
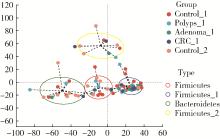
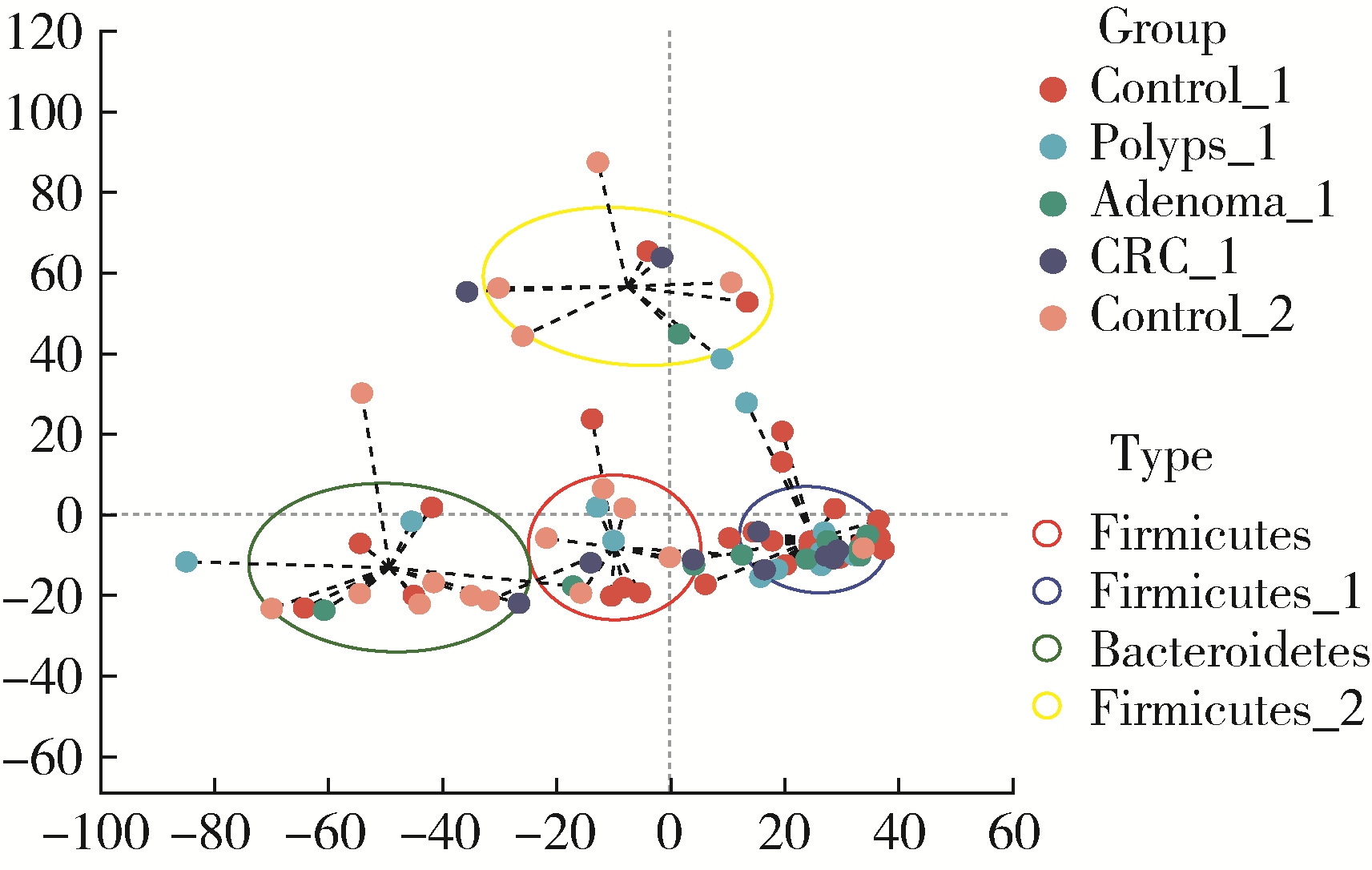
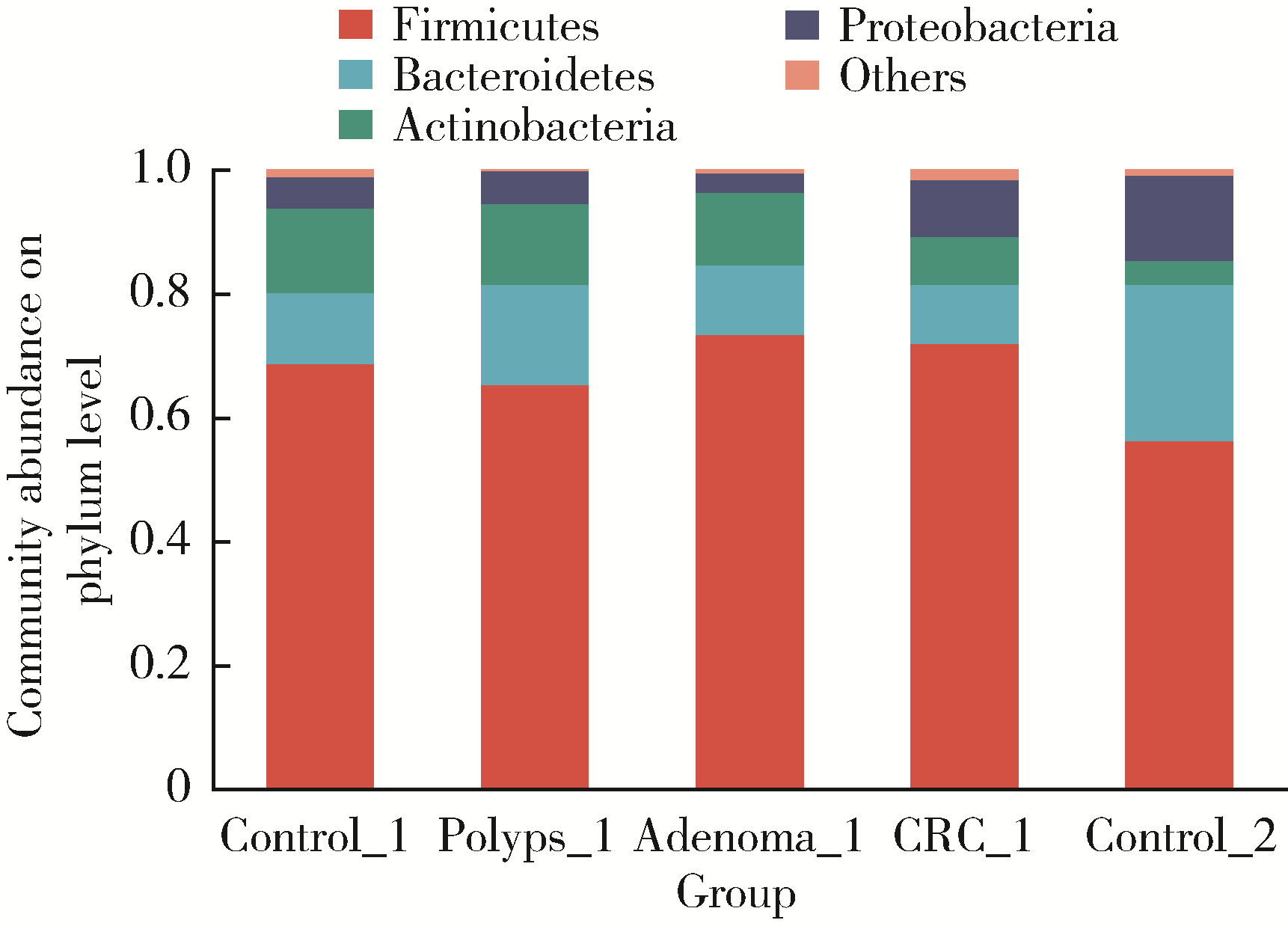
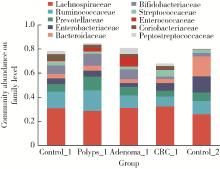
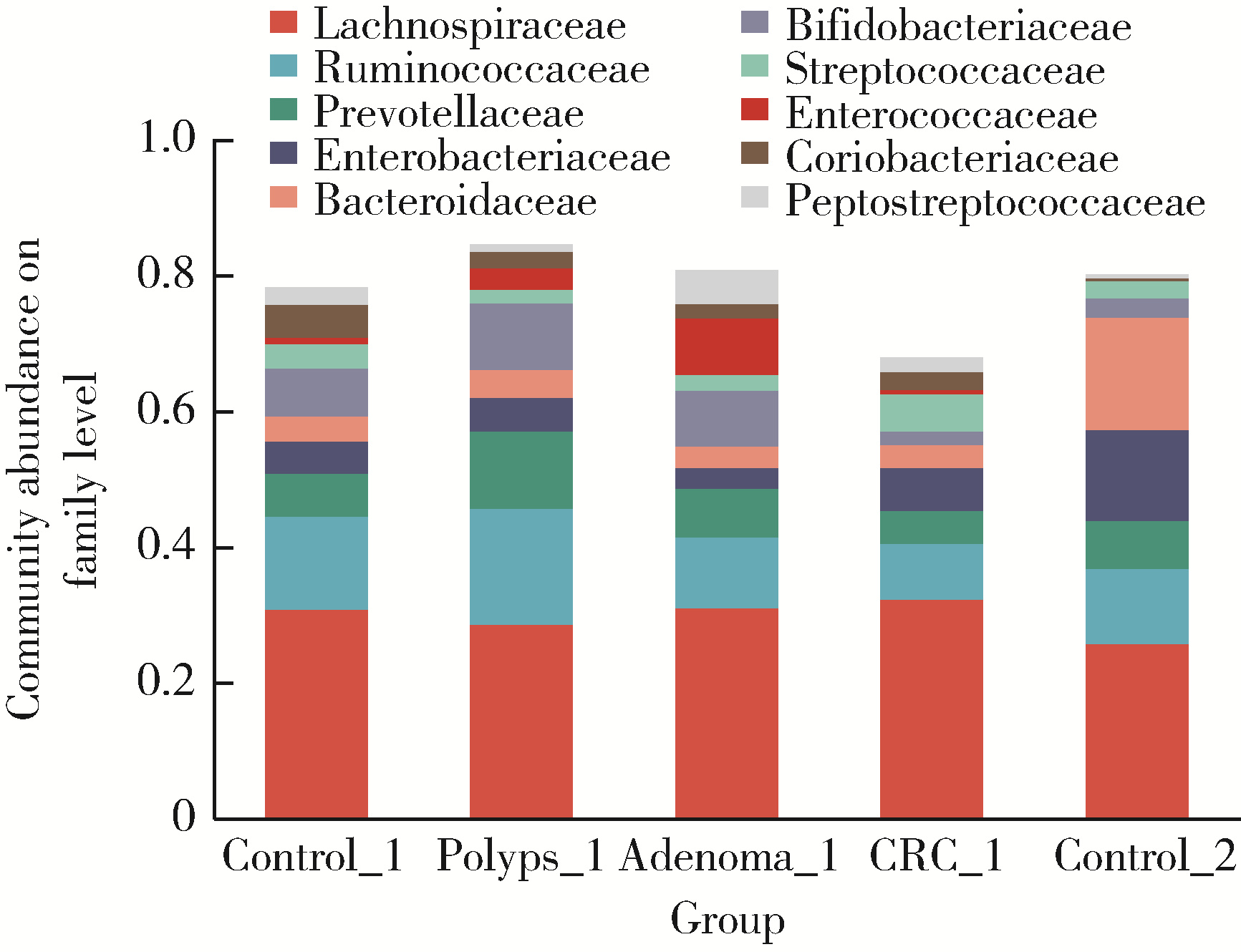
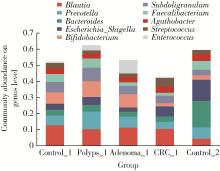
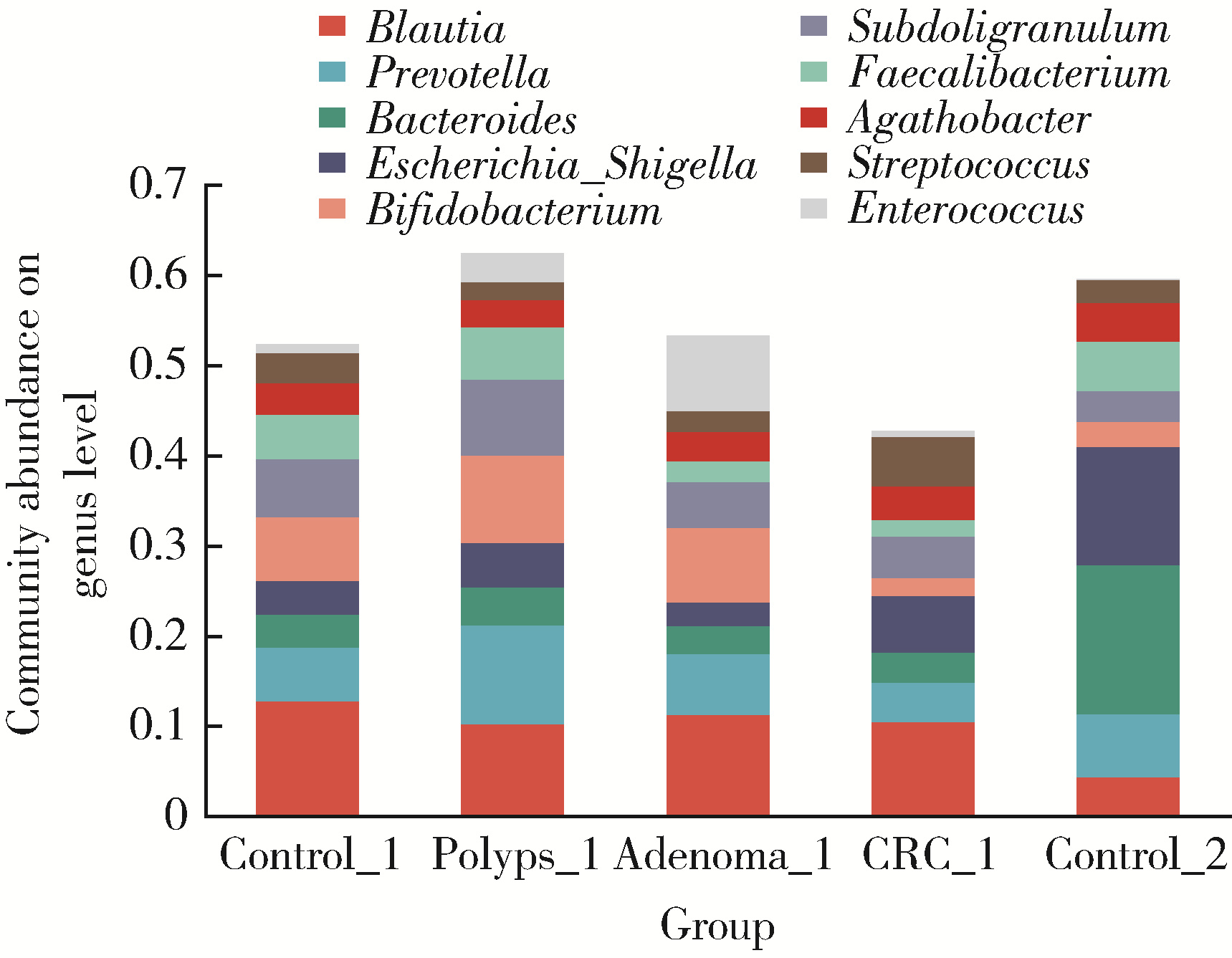
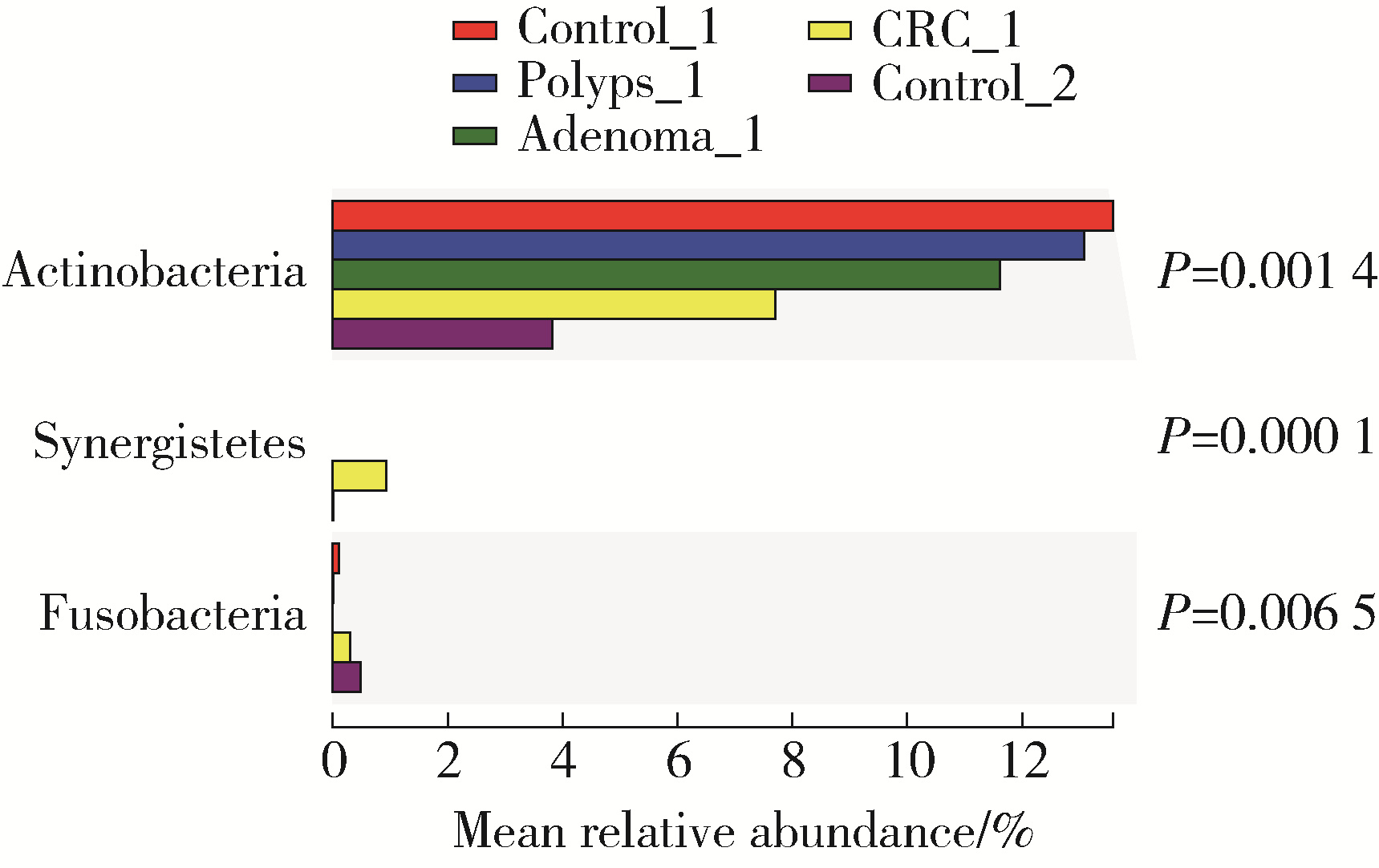
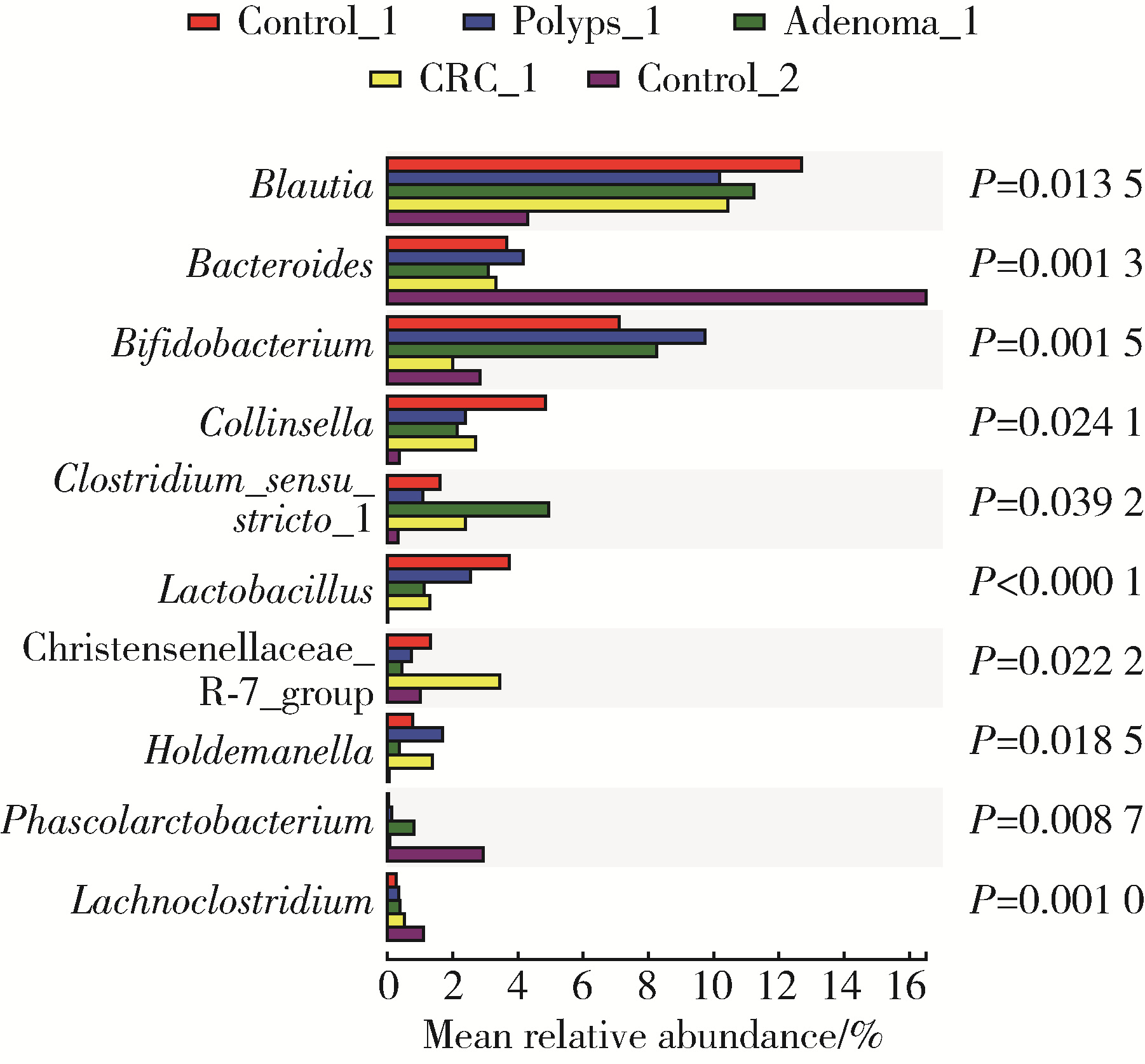
目的: 分析高海拔地区结直肠良恶性肿瘤患者肠道菌群变化,并与低海拔地区人群进行比较。方法: 收集2020—2022年于西藏自治区人民医院接受结肠镜检查的61例患者的临床资料,根据结肠镜检查结果分为4组高海拔对照组(29例)、非腺瘤性息肉组(12例)、腺瘤组(10例)和结直肠癌组(10例),收集同期在北京大学第三医院接受结肠镜检查且结果正常者17例为低海拔对照组。结肠镜检查肠道准备前收取受试者的粪便标本,进行细菌DNA提取,对16S rRNA基因V3~V4可变区进行PCR扩增及高通量测序,得到粪便样本的菌群组成,进行物种的多样性分析。结果: α多样性分析显示,高海拔结直肠癌组样本物种多样性与高海拔非腺瘤性息肉组及低海拔对照组间差异有统计学意义,且高海拔结直肠癌组样本的物种多样性较其他两组高;但5组间的β多样性差异无统计学意义。门水平的差异分析发现,低海拔对照组中放线菌门(Actinobacteria)丰度明显低于高海拔各组,而高海拔结直肠癌组放线菌门丰度明显低于其他3个高海拔组。属水平的差异分析发现,低海拔对照组中拟杆菌属(Bacteroides)、考拉杆菌属(Phascolarctobacterium)、梭状芽胞杆菌属(Lachnoclostridium)丰度明显较所有高海拔组高;布鲁菌属(Blautia)、科林斯菌属(Collinsella)在高海拔对照组中丰度最高;低海拔对照组中未检测到乳酸杆菌属(Lactobacillus),而高海拔各组间乳酸杆菌属丰度差异有统计学意义(P≤0.001),且高海拔对照组明显高于其他3组;高海拔结直肠癌组双歧杆菌属(Bifidobacterium)丰度明显下降,但克里斯滕森氏菌科R-7群(Christensenellaceae_R-7_group)丰度明显升高。结论: 与高海拔及低海拔对照组相比,高海拔结直肠良恶性肿瘤患者肠道菌群的多样性及丰富度存在差异,且在门及属水平上的物种丰度也存在差异,表明海拔因素可能对肠道菌群有一定的影响。
中图分类号:
- R574.62
| 1 |
doi: 10.3390/genes11070808 |
| 2 |
刘贵琴, 李向阳. 高原低氧条件下的肠道菌群与药物代谢[J]. 药学研究, 2019, 38 (12): 714- 718.
|
| 3 |
doi: 10.1007/s00253-015-7039-6 |
| 4 |
doi: 10.1093/bioinformatics/btr507 |
| 5 |
doi: 10.1038/nmeth.2604 |
| 6 |
doi: 10.1099/00207713-44-4-846 |
| 7 |
doi: 10.1007/s12263-011-0229-7 |
| 8 |
doi: 10.3389/fmicb.2017.01929 |
| 9 |
梁田, 马利锋, 张致英, 等. 海拔高度与藏族人群肠道菌群的宏基因组学关联分析[J]. 天津师范大学学报(自然科学版), 2021, 41 (2): 36- 43.
|
| 10 |
doi: 10.1101/gr.126573.111 |
| 11 |
doi: 10.1371/journal.pone.0155863 |
| 12 |
doi: 10.1038/srep14682 |
| 13 |
于鑫, 张永镇, 于恩达, 等. 结直肠腺瘤与息肉患者肠道菌群变化的病例对照研究[J]. 复旦学报(医学版), 2018, 45 (5): 658- 663.
|
| 14 |
蔡平. 大肠癌患者肠道菌群多样性特征及驱动菌种鉴定和功能预测研究[D]. 浙江: 宁波大学, 2020.
|
| 15 |
宋丽, 陈力军, 王红美, 等. 双歧三联活菌片对甲氨蝶呤所致大鼠黏膜炎的干预作用[J]. 实用儿科临床杂志, 2009, 24 (15): 1176- 1178.
|
| 16 |
|
| 17 |
doi: 10.1002/ibd.20525 |
| 18 |
doi: 10.1097/MOG.0b013e32832b20bf |
| 19 |
单体栋, 邓芝云, 张方信, 等. 急进高原与平原大鼠肠道双歧杆菌的分子生物学实验对比研究[J]. 中国微生态学杂志, 2011, 23 (3): 197- 200.
|
| 20 |
余倩, 周蓉, 裴晓方, 等. 双歧杆菌活菌液对肠道菌群调节效果的研究[J]. 现代预防医学, 2008, 35 (20): 4038- 4040.
|
| [1] | 梁丽,李鑫,农琳,董颖,张继新,李东,李挺. 子宫内膜癌微卫星不稳定性分析: 微小微卫星变换的意义[J]. 北京大学学报(医学版), 2023, 55(2): 254-261. |
| [2] | 丁婷婷,曾楚雄,胡丽娜,余明华. 基于癌症基因组图谱数据库结直肠癌免疫细胞浸润预测模型的建立[J]. 北京大学学报(医学版), 2022, 54(2): 203-208. |
| [3] | 牛占岳,薛艳,张静,张贺军,丁士刚. 胃腺瘤性息肉的内镜和病理特点及癌变的危险因素分析[J]. 北京大学学报(医学版), 2021, 53(6): 1122-1127. |
| [4] | 董博,马晓伟,郭晓蕙,高莹,张俊清. 卡托普利试验在醛固酮腺瘤无创诊断中的临床价值[J]. 北京大学学报(医学版), 2021, 53(6): 1128-1132. |
| [5] | 安文成,闫慧娴,邓正照,陈芳,欧小虹,金红心,黄薇. 原发性醛固酮增多症肾上腺切除术后慢性肾功能不全1例[J]. 北京大学学报(医学版), 2021, 53(6): 1201-1204. |
| [6] | 邱敏,费月阳,邓绍晖,刘承,卢剑,何为,陆敏,田晓军,张树栋,马潞林. 后肾腺瘤的诊治经验及文献回顾[J]. 北京大学学报(医学版), 2021, 53(2): 417-419. |
| [7] | 王迎春,黄永辉,常虹,姚炜,闫秀娥,李柯,张耀鹏,郑炜. 十二指肠乳头息肉良、恶性病变比较及活检准确性[J]. 北京大学学报(医学版), 2021, 53(1): 204-209. |
| [8] | 康琦,张继新,高莹,张俊清,郭晓蕙. 100例甲状腺嗜酸细胞腺瘤的诊治分析[J]. 北京大学学报(医学版), 2020, 52(6): 1098-1101. |
| [9] | 王骁,李兆星,范焕芳,魏莉瑛,郭旭瑾,郭娜,王彤. 罕见小肠囊腺瘤1例报道[J]. 北京大学学报(医学版), 2020, 52(2): 382-384. |
| [10] | 张旭初,张建华,王荣福,范岩,付占立,闫平,赵光宇,白艳霞. 18F-FDG PET/CT联合多种肿瘤标志物在结直肠中分化腺癌术后复发及转移中的应用价值[J]. 北京大学学报(医学版), 2019, 51(6): 1071-1077. |
| [11] | 叶剑飞,王冰,马潞林,赵磊,王国良,洪锴. 保留器官的睾丸部分切除术治疗睾丸腺瘤样瘤[J]. 北京大学学报(医学版), 2019, 51(2): 365-368. |
| [12] | 杨文博,张晓威,杨健,李清,徐涛,白文俊. 经尿道前列腺电切术治疗复发性前列腺囊腺瘤1例[J]. 北京大学学报(医学版), 2018, 50(4): 740-742. |
| [13] | 陈杨,王艳荣,石燕,戴广海. 晚期结直肠癌患者一线应用FOLFOX方案化疗引起中性粒细胞减少的预后价值[J]. 北京大学学报(医学版), 2017, 49(4): 669-674. |
| [14] | 刘艳霞,杨雪松,付卫,姚宏伟. 单核苷酸多态性位点rs6983267与溃疡性结肠炎及大肠癌的相关性[J]. 北京大学学报(医学版), 2016, 48(6): 994-999. |
| [15] | 席晨光,范宇,杨新宇,刘漓波,王静华,胡帅,李妍妍,何群. 16例后肾腺瘤的临床病理特点及鉴别诊断[J]. 北京大学学报(医学版), 2016, 48(4): 598-602. |
|
||