北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1122-1127. doi: 10.19723/j.issn.1671-167X.2021.06.019
胃腺瘤性息肉的内镜和病理特点及癌变的危险因素分析
- 北京大学第三医院消化科,北京 100191
Analysis of endoscopic and pathological features of gastric adenomatous polyps and risk factors for canceration
NIU Zhan-yue,XUE Yan,ZHANG Jing,ZHANG He-jun,DING Shi-gang( )
)
- Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China
摘要:
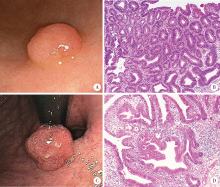
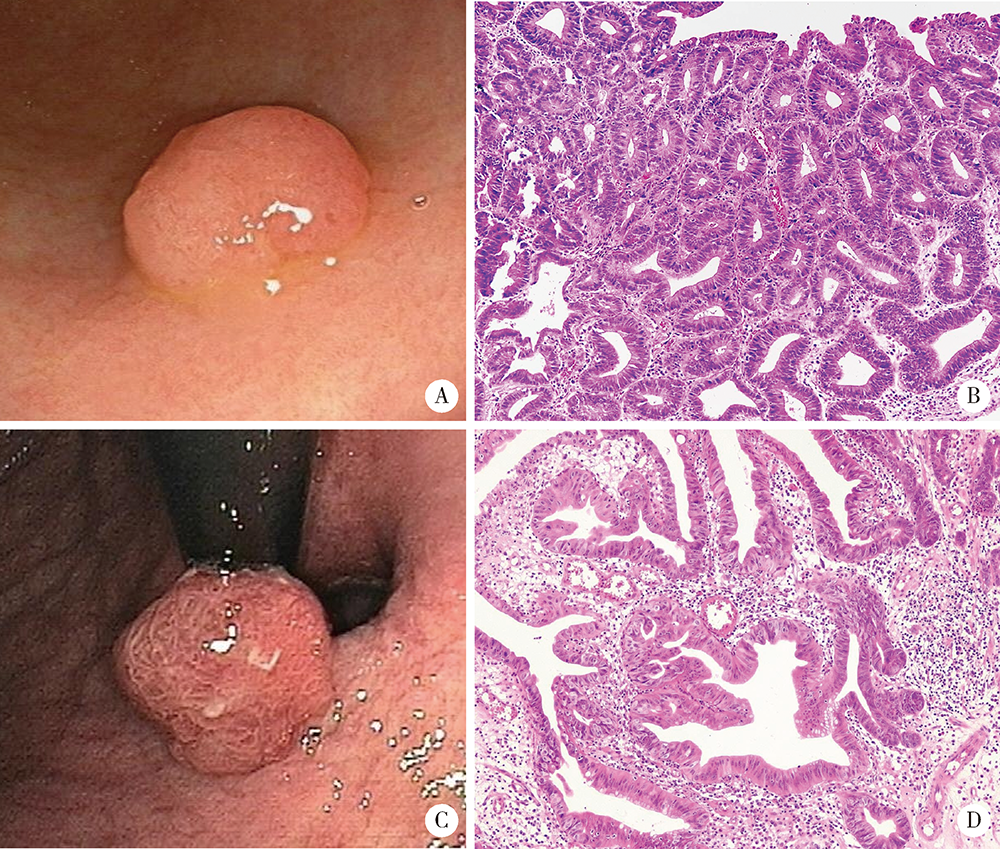
目的:分析胃腺瘤性息肉的内镜和病理特点,探讨其癌变的危险因素。方法:回顾性总结北京大学第三医院2005年1月1日至2019年12月31日的胃腺瘤性息肉患者的内镜和病理特点,并分析癌变的危险因素。结果:共纳入胃腺瘤性息肉患者125例,女性占51.20%,平均年龄为(66.7±12.3)岁,≥65岁的患者占64.80%,<45岁者仅占5.60%。腺瘤性息肉多分布在胃体和胃窦,分别占40.80%和32.80%,以单发(90.40%)、无蒂(76.81%)为主。65.94%的腺瘤性息肉直径≤1.0 cm。23.20%(29/125)的患者合并增生性息肉和/或胃底腺息肉,其中,合并多发息肉者占58.62%(17/29),1.60%(2/125)的患者同时存在两种病理类型的息肉,1.60%(2/125)的患者伴发G1期的胃神经内分泌肿瘤。13.60%(17/125)的腺瘤性息肉患者伴发胃癌,以进展期(70.59%)、未分化型(66.67%)癌为主。伴发低级别上皮内瘤变者为18.40%(23/125)。52.80%的背景胃黏膜是慢性萎缩性胃炎伴肠化生,自身免疫性胃炎占11.20%。幽门螺杆菌的阳性率为21.60%。胃腺瘤性息肉的癌变率为20.80%(26/125),癌变以分化型为主,但有的癌变为印戒细胞癌。直径>1.0 cm(OR=5.092,95%CI: 1.447~17.923,P=0.011)、表面形态不平伴有糜烂(OR=13.749,95%CI: 1.072~176.339,P=0.044)是癌变的独立危险因素。结论:胃腺瘤性息肉伴发胃癌的比例高、癌变率高,直径和表面形态是癌变的独立危险因素。内镜检查时应重视内镜下息肉病理类型的鉴别,并重视全胃黏膜的评估。
中图分类号:
- R735.2
| [1] |
Carmack SW, Genta RM, Schuler CM, et al. The current spectrum of gastric polyps: A 1-year national study of over 120,000 patients[J]. Am J Gastroenterol, 2009, 104(6):1524-1532.
doi: 10.1038/ajg.2009.139 |
| [2] |
Corral JE, Keihanian T, Diaz LI, et al. Management patterns of gastric polyps in the United States[J]. Frontline Gastroenterol, 2019, 10(1):16-23.
doi: 10.1136/flgastro-2017-100941 |
| [3] |
Enestvedt BK, Chandrasekhara V, Ginsberg GG. Endoscopic ultrasonographic assessment of gastric polyps and endoscopic mucosal resection[J]. Curr Gastroenterol Rep, 2012, 14(6):497-503.
doi: 10.1007/s11894-012-0292-2 pmid: 23001857 |
| [4] |
Velázquez-Dohorn ME, López-Durand CF, Gamboa-Domínguez A. Changing trends in gastric polyps[J]. Rev Invest Clin, 2018, 70(1):40-45.
doi: 10.24875/RIC.17002430 pmid: 29513301 |
| [5] |
Castro R, Pimentel-Nunes P, Dinis-Ribeiro M. Evaluation and management of gastric epithelial polyps[J]. Best Pract Res Clin Gastroenterol, 2017, 31(4):381-387.
doi: S1521-6918(17)30060-4 pmid: 28842047 |
| [6] |
Chen WC, Wallace MB. Endoscopic management of mucosal lesions in the gastrointestinal tract[J]. Expert Rev Gastroenterol Hepatol, 2016, 10(4):481-495.
doi: 10.1586/17474124.2016.1122520 |
| [7] | Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma[J]. Gut, 2019, 68(9):1545-1575. |
| [8] |
Laxén F, Sipponen P, Iham?ki T. Gastric polyps: their morphological and endoscopical characteristics and relation to gastric carcinoma[J]. Acta Pathol Microbiol Immunol Scand A, 1982, 90(3):221-228.
pmid: 7102316 |
| [9] |
Borch K, Skarsgard J, Franzen L, et al. Benign gastric polyps: Morphological and functional origin[J]. Dig Dis Sci, 2003, 48(7):1292-1297.
doi: 10.1023/A:1024150924457 |
| [10] |
Zhao G, Xue M, Hu Y, et al. How commonly is the diagnosis of gastric low grade dysplasia upgraded following endoscopic resection? A meta-analysis[J]. PLoS One, 2015, 10(7):e0132699.
doi: 10.1371/journal.pone.0132699 |
| [11] |
de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands[J]. Gastroenterology, 2008, 134(4):945-952.
doi: 10.1053/j.gastro.2008.01.071 |
| [12] |
Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer[J]. J Clin Pathol, 2002, 55(10):770-773.
pmid: 12354805 |
| [13] |
Zhang H, Jin Z, Cui R, et al. Autoimmune metaplastic atrophic gastritis in chinese: A study of 320 patients at a large tertiary medical center[J]. Scand J Gastroenterol, 2017, 52(2):150-156.
doi: 10.1080/00365521.2016.1236397 |
| [14] |
Zhang H, Nie X, Song Z, et al. Hyperplastic polyps arising in autoimmune metaplastic atrophic gastritis patients: Is this a distinct clinicopathological entity[J]. Scand J Gastroenterol, 2018, 53(10/11):1186-1193.
doi: 10.1080/00365521.2018.1514420 |
| [15] |
Suzuki S, Gotoda T, Suzuki H, et al. Morphologic and histologic changes in gastric adenomas after Helicobacter pylori eradication: A long-term prospective analysis[J]. Helicobacter, 2015, 20(6):431-437.
doi: 10.1111/hel.12218 pmid: 25704290 |
| [16] |
Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht V/Florence Consensus Report[J]. Gut, 2017, 66(1):6-30.
doi: 10.1136/gutjnl-2016-312288 pmid: 27707777 |
| [17] |
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endosco-pic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline[J]. Endoscopy, 2015, 47(9):829-854.
doi: 10.1055/s-0034-1392882 pmid: 26317585 |
| [1] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [2] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [3] | 黄教悌,胡菁,韩博. 治疗相关神经内分泌前列腺癌机制研究与靶向治疗新进展[J]. 北京大学学报(医学版), 2024, 56(4): 557-561. |
| [4] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [5] | 姚凯烽,阮明健,李德润,田宇轩,陈宇珂,范宇,刘毅. 靶向穿刺联合区域系统穿刺对PI-RADS 4~5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2024, 56(4): 575-581. |
| [6] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [7] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [8] | 王滨帅,邱敏,张前进,田茂锋,刘磊,王国良,陆敏,田晓军,张树栋. 6例肾尤文肉瘤伴静脉瘤栓的诊治[J]. 北京大学学报(医学版), 2024, 56(4): 636-639. |
| [9] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [10] | 舒帆,郝一昌,张展奕,邓绍晖,张洪宪,刘磊,王国良,田晓军,赵磊,马潞林,张树栋. 肾部分切除术治疗囊性肾癌的功能学和肿瘤学结果:单中心回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 667-672. |
| [11] | 陈延,李况蒙,洪锴,张树栋,程建星,郑仲杰,唐文豪,赵连明,张海涛,姜辉,林浩成. 阴茎海绵体注射试验对阴茎血管功能影响的回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 680-686. |
| [12] | 庞博,郭桐君,陈曦,郭华棋,石嘉章,陈娟,王欣梅,李耀妍,单安琪,余恒意,黄婧,汤乃军,王艳,郭新彪,李国星,吴少伟. 天津与上海35岁以上人群氮氧化物个体暴露水平及其影响因素[J]. 北京大学学报(医学版), 2024, 56(4): 700-707. |
| [13] | 和静,房中则,杨颖,刘静,马文瑶,霍勇,高炜,武阳丰,谢高强. 血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系[J]. 北京大学学报(医学版), 2024, 56(4): 722-728. |
| [14] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [15] | 蔡珊,张依航,陈子玥,刘云飞,党佳佳,师嫡,李佳欣,黄天彧,马军,宋逸. 北京市中小学生身体活动时间现状及影响因素的路径[J]. 北京大学学报(医学版), 2024, 56(3): 403-410. |
|
||