北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (4): 684-691. doi: 10.19723/j.issn.1671-167X.2025.04.009
基于临床特征和多参数MRI的前列腺癌盆腔淋巴结转移的术前预测模型
王泽远, 于栓宝, 郑浩轲, 陶金, 范雅峰, 张雪培*( )
)
- 郑州大学第一附属医院泌尿外科, 郑州 450000
A preoperative prediction model for pelvic lymph node metastasis in prostate cancer: Integrating clinical characteristics and multiparametric MRI
Zeyuan WANG, Shuanbao YU, Haoke ZHENG, Jin TAO, Yafeng FAN, Xuepei ZHANG*( )
)
- Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
摘要:
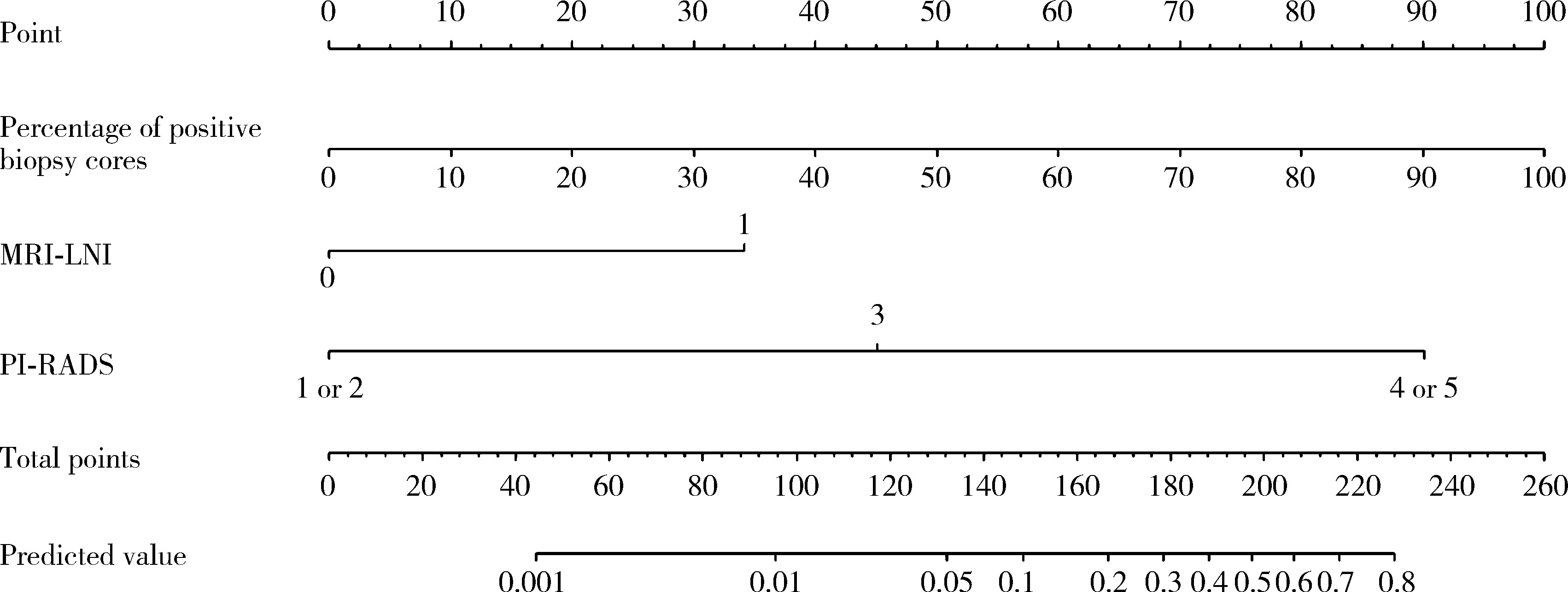
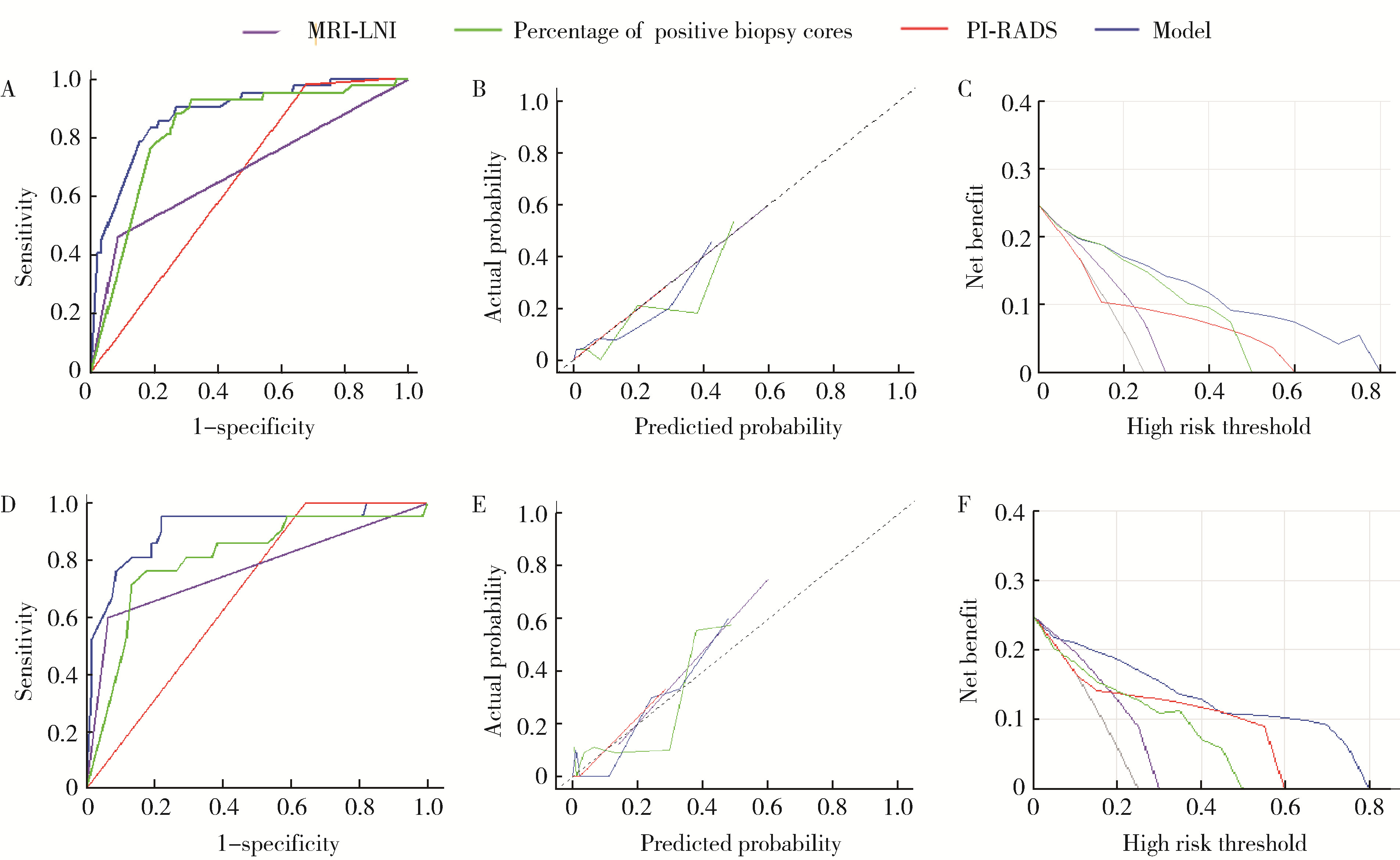
目的: 分析与前列腺癌患者盆腔淋巴结转移(pelvic lymph node metastasis, PLNM)相关的临床特征, 构建PLNM的术前预测模型, 以减少不必要的扩大盆腔淋巴结清扫(extended pelvic lymph node dissection, ePLND)。方法: 根据纳入与排除标准, 回顾性收集2014—2024年间在郑州大学第一附属医院接受前列腺癌根治术和ePLND的344例患者, 其中77例(22.4%)患者淋巴结阳性。收集患者的临床特征、MRI报告和组织病理结果, 将数据随机分为训练集(241例, 70%)和验证集(103例, 30%), 采用单因素和多因素Logistic回归分析构建PLNM的术前预测模型。结果: 单因素Logistic回归分析表明, 总前列腺特异性抗原(total prostate specific antigen, tPSA) (P=0.021)、游离前列腺特异性抗原(free prostate specific antigen, fPSA) (P=0.002)、fPSA/tPSA (P=0.011)、穿刺阳性针数百分比(P < 0.001)、前列腺影像报告和数据系统(prostate imaging reporting and data system, PI-RADS)评分(P=0.004)、穿刺病理Gleason评分≥8 (P=0.005)、临床T分期(P < 0.001)和MRI显示的淋巴结受累(MRI-indicated lymph node involvement, MRI-LNI) (P < 0.001)是预测PLNM的显著因素。多因素Logistic回归分析表明, 穿刺阳性针数百分比(OR=91.24, 95%CI: 13.34~968.68)、PI-RADS评分(OR=7.64, 95%CI: 1.78~138.06)和MRI-LNI (OR=4.67, 95%CI: 1.74~13.24)是预测PLNM的独立危险因素。基于此构建列线图, 多因素模型的预测效果[曲线下面积(area under curve, AUC)=0.883]显著优于单一指标[阳性针数百分比(AUC=0.806)、PI-RADS评分(AUC=0.679)和MRI-LNI(AUC=0.768)]。校准曲线和决策曲线表明, 多因素模型具有较高的预测准确度和显著的净收益, 在6%的截断值下只漏检了约5.2%的PLNM(4/77), 而减少了约53%的ePLND(139/267), 显示出较好的预测效果。结论: 穿刺阳性针数百分比、PI-RADS评分和MRI-LNI是PLNM的独立危险因素, 构建多因素模型可显著提高预测效果, 为指导临床ePLND策略提供了有价值的参考。
中图分类号:
- R737.25
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| [1] | 宁家昕, 王浩然, 罗书航, 敬吉波, 王建业, 侯惠民, 刘明. 氧化应激相关基因与前列腺癌关系的多组学分析[J]. 北京大学学报(医学版), 2025, 57(4): 633-643. |
| [2] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [3] | 邢念增,王明帅,杨飞亚,尹路,韩苏军. 前列腺免活检创新理念的临床实践及其应用前景[J]. 北京大学学报(医学版), 2024, 56(4): 565-566. |
| [4] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [5] | 姚凯烽,阮明健,李德润,田宇轩,陈宇珂,范宇,刘毅. 靶向穿刺联合区域系统穿刺对PI-RADS 4~5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2024, 56(4): 575-581. |
| [6] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [7] | 薛蔚,董樑,钱宏阳,费笑晨. 前列腺癌新辅助治疗与辅助治疗的现状及进展[J]. 北京大学学报(医学版), 2023, 55(5): 775-780. |
| [8] | 刘毅,袁昌巍,吴静云,沈棋,肖江喜,赵峥,王霄英,李学松,何志嵩,周利群. 靶向穿刺+6针系统穿刺对PI-RADS 5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2023, 55(5): 812-817. |
| [9] | 毛海,张帆,张展奕,颜野,郝一昌,黄毅,马潞林,褚红玲,张树栋. 基于MRI前列腺腺体相关参数构建腹腔镜前列腺癌术后尿失禁的预测模型[J]. 北京大学学报(医学版), 2023, 55(5): 818-824. |
| [10] | 袁昌巍,李德润,李志华,刘毅,山刚志,李学松,周利群. 多参数磁共振成像中动态对比增强状态在诊断PI-RADS 4分前列腺癌中的应用[J]. 北京大学学报(医学版), 2023, 55(5): 838-842. |
| [11] | 田聪,刘军,杨波,乔佳佳,黄晓波,许清泉. 经皮肾镜取石术中异常肾盂黏膜活检结果分析[J]. 北京大学学报(医学版), 2023, 55(5): 948-952. |
| [12] | 郑丹枫,李君禹,李佳曦,张英爽,钟延丰,于淼. 青少年特发性脊柱侧凸椎旁肌的病理特征[J]. 北京大学学报(医学版), 2023, 55(2): 283-291. |
| [13] | 武颖超,蔡云龙,戎龙,张继新,刘金,汪欣. 早期胃癌淋巴结转移规律及内镜黏膜下剥离术治疗早期胃癌的疗效评价[J]. 北京大学学报(医学版), 2020, 52(6): 1093-1097. |
| [14] | 刘毅,刘志坚,沈棋,吴静云,范宇,李德润,虞巍,何志嵩. 14例恶性潜能未定的前列腺间质肿瘤病例分析[J]. 北京大学学报(医学版), 2020, 52(4): 621-624. |
| [15] | 郝一昌,颜野,张帆,邱敏,周朗,刘可,卢剑,肖春雷,黄毅,刘承,马潞林. 穿刺活检单针阳性的前列腺癌手术策略选择及经验总结[J]. 北京大学学报(医学版), 2020, 52(4): 625-631. |
|
||