Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (4): 730-737. doi: 10.19723/j.issn.1671-167X.2020.04.026
Previous Articles Next Articles
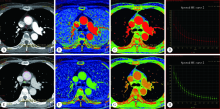
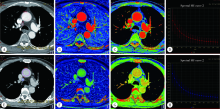
Comparative imaging study of mediastinal lymph node from pre-surgery dual energy CT versus post-surgeron verifications in non-small cell lung cancer patients
Qiao ZHU,Cui REN,Yan ZHANG,Mei-jiao LI,Xiao-hua WANG( )
)
- Department of Radiology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R734.2
| [1] | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018,68(1):7-30. |
| [2] |
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods[J]. Int J Cancer, 2019,144(8):1941-1953.
doi: 10.1002/ijc.31937 pmid: 30350310 |
| [3] | 中华医学会, 中华医学会肿瘤学分会, 中华医学会杂志社. 中华医学会肺癌临床诊疗指南(2018版)[J]. 中华肿瘤杂志, 2018,40(12):935-964. |
| [4] |
Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging[J]. J Nucl Med, 2004,45(9):1509-1518.
pmid: 15347718 |
| [5] | Vansteenkiste J, Dooms C, De Leyn P. Early stage non-small cell lung cancer: challenges in staging and adjuvant treatment: evidence-based staging[J]. Ann Oncol, 2010, 21(Suppl 7): vii189-vii195. |
| [6] | 宁先英, 李浩, 杨明, 等. CT能谱定量分析对肺腺癌与鳞癌的鉴别诊断价值[J]. 放射学实践, 2017,32(3):237-241. |
| [7] |
Lv P, Lin XZ, Li J, et al. Differentiation of small hepatic hemangioma from small hepatocellular carcinoma: recently introduced spectral CT method[J]. Radiology, 2011,259(3):720-729.
doi: 10.1148/radiol.11101425 pmid: 21357524 |
| [8] |
Martin SS, Weidinger S, Czwikla R, et al. Iodine and fat quantification for differentiation of adrenal gland adenomas from metas-tases using third-generation dual-source dual-energy computed tomography[J]. Invest Radiol, 2018,53(3):173-178.
pmid: 28990974 |
| [9] |
Muenzel D, Lo GC, Yu HS, et al. Material density iodine images in dual-energy CT: detection and characterization of hypervascular liver lesions compared to magnetic resonance imaging[J]. Eur J Radiol, 2017,95(10):300-306.
doi: 10.1016/j.ejrad.2017.08.035 |
| [10] |
Liu X, Ouyang D, Li H, et al. Papillary thyroid cancer: dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes[J]. Radiology, 2015,275(1):167-176.
pmid: 25521777 |
| [11] |
De Leyn P, Vansteenkiste J, Cuypers P, et al. Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan[J]. Eur J Cardiothorac Surg, 1997,12(5):706-712.
doi: 10.1016/s1010-7940(97)00253-4 pmid: 9458140 |
| [12] |
Fukuya T, Honda H, Hayashi T, et al. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer[J]. Radiology, 1995,197(3):705-711.
pmid: 7480743 |
| [13] |
Yoshimura G, Sakurai T, Oura S, et al. Evaluation of axillary lymph node status in breast cancer with MRI[J]. Breast Cancer, 1999,6(3):249-258.
pmid: 11091725 |
| [14] |
Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: an initial experience[J]. Eur J Radiol, 2016,85(6):1219-1223.
doi: 10.1016/j.ejrad.2016.03.030 pmid: 27161073 |
| [15] |
Rizzo S, Radice D, Femia M, et al. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT[J]. Eur Radiol, 2018,28(2):760-769.
doi: 10.1007/s00330-017-5015-5 pmid: 28835993 |
| [16] | Yang Z, Zhang X, Fang M, et al. Preoperative diagnosis of regional lymph node metastasis of colorectal cancer with quantitative parameters from dual-energy CT[J]. AJR Am J Roentgenol, 2019,213(6):1-9. |
| [17] |
Zhang X, Zheng C, Yang Z, et al. Axillary sentinel lymph nodes in breast cancer: quantitative evaluation at dual-energy CT[J]. Radiology, 2018,289(2):337-346.
doi: 10.1148/radiol.2018180544 pmid: 30152748 |
| [18] | Yang F, Dong J, Wang X, et al. Non-small cell lung cancer: spectral computed tomography quantitative parameters for preoperative diagnosis of metastatic lymph nodes[J]. Eur J Radiol, 2017,89(4):129-135. |
| [19] |
Lin LY, Zhang Y, Suo ST, et al. Correlation between dual-energy spectral CT imaging parameters and pathological grades of non-small cell lung cancer[J]. Clin Radiol, 2018, 73(4): 412.e1-412.e7.
doi: 10.1016/j.crad.2017.10.020 pmid: 29195660 |
| [20] | 崔元龙, 许毛荣, 文智. 能谱CT定量参数对非小细胞肺癌纵隔淋巴结转移中的应用价值[J]. 临床放射学杂志, 2019,38(5):825-829. |
| [21] | 叶亚君, 莫淑琼. CT能谱成像鉴别诊断纵隔淋巴结良恶性的临床意义[J]. 现代医用影像学, 2018,27(8):2702-2703. |
| [22] |
Pan Z, Pang L, Ding B, et al. Gastric cancer staging with dual energy spectral CT imaging[J]. PLoS One, 2013,8(2):e53651.
doi: 10.1371/journal.pone.0053651 pmid: 23424614 |
| [23] | Yang L, Luo D, Li L, et al. Differentiation of malignant cervical lymphadenopathy by dual-energy CT: a preliminary analysis[J]. Sci Rep, 2016,6(1):31020. |
| [1] | Hua ZHONG, Yuan LI, Liling XU, Mingxin BAI, Yin SU. Application of 18F-FDG PET/CT in rheumatic diseases [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 853-859. |
| [2] | Shishi BO,Chengzhi GAO. Tooth segmentation and identification on cone-beam computed tomography with convolutional neural network based on spatial embedding information [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 735-740. |
| [3] | Xiaotong LING,Liuyang QU,Danni ZHENG,Jing YANG,Xuebing YAN,Denggao LIU,Yan GAO. Three-dimensional radiographic features of calcifying odontogenic cyst and calcifying epithelial odontogenic tumor [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 131-137. |
| [4] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [5] | Xiang LIU,Hui-hui XIE,Yu-feng XU,Xiao-dong ZHANG,Xiao-feng TAO,Lin LIU,Xiao-ying WANG. Value of artificial intelligence in the improvement of diagnostic consistency of radiology residents [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 670-675. |
| [6] | Jin-hua ZHANG,Jie PAN,Zhi-peng SUN,Xiao WANG. Effect of various intracanal materials on the diagnostic accuracy of cone-beam computed tomography in vertical root fractures [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 333-338. |
| [7] | Jia-xue YE,Yu-hong LIANG. A prevalence survey of cone-beam computed tomography use among endodontic practitioners [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 114-119. |
| [8] | Meng-qiao PAN,Jian LIU,Li XU,Xiao XU,Jian-xia HOU,Xiao-tong LI,Xiao-xia WANG. A long-term evaluation of periodontal phenotypes before and after the periodontal-orthodontic-orthognathic combined treatment of lower anterior teeth in patients with skeletal Angle class Ⅲ malocclusion [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 52-61. |
| [9] | Yu FU,Xin-nong HU,Sheng-jie CUI,Jie SHI. Decompensation effectiveness and alveolar bone remodeling analysis of mandibular anterior teeth after preoperative orthodontic treatment in high-angle patients with skeletal class Ⅱ malocclusion [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 62-69. |
| [10] | Yu WANG,Hui-min ZHANG,Xue-rong DENG,Wei-wei LIU,Lu CHEN,Ning ZHAO,Xiao-hui ZHANG,Zhi-bo SONG,Yan GENG,Lan-lan JI,Yu WANG,Zhuo-li ZHANG. Diagnostic values of urinary citrate for kidney stones in patients with primary gout [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1134-1140. |
| [11] | Juan GAO,Hang-miao LV,Hui-min MA,Yi-jiao ZHAO,Xiao-tong LI. Evaluation of root resorption after surgical orthodontic treatment of skeletal Class Ⅲ malocclusion by three-dimensional volumetric measurement with cone-beam CT [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 719-726. |
| [12] | WANG Shu-lei,GAO Yang-xu,ZHANG Hong-wu,YANG Hai-bo,LI Hui,LI Yu,SHEN Li-xue,YAO Hong-xin. Clinical analysis of 30 cases of basal ganglia germinoma in children [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 222-226. |
| [13] | LIU Wei-tao,WANG Yi-ran,WANG Xue-dong,ZHOU Yan-heng. A cone-beam computed tomography evaluation of three-dimensional changes of circummaxillary sutures following maxillary protraction with alternate rapid palatal expansions and constrictions [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 346-355. |
| [14] | Gang YANG,Wen-jie HU,Jie CAO,Deng-gao LIU. Three-dimensional morphology analysis of the supraosseous gingival profile of periodontally healthy maxillary anterior teeth [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 990-994. |
| [15] | LI Xin-fei, PENG Yi-ji, YU Xiao-teng, XIONG Sheng-wei, CHENG Si-da, DING Guang-pu, YANG Kun-lin, TANG Qi, MI Yue, WU Jing-yun, ZHANG Peng, XIE Jia-xin, HAO Han, WANG He, QIU Jian-xing, YANG Jian, LI Xue-song, ZHOU Li-qun. Three dimensional nephrometry system for partial nephrectomy: Our initial exploration [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 613-622. |
|
||