北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (3): 477-486. doi: 10.19723/j.issn.1671-167X.2019.03.015
紫杉醇微球-原位凝胶的制备及其局部注射的抗肿瘤药效
Preparation and characterization of paclitaxel microspheres in situ gel and its antitumor efficacy by local injection
Ying ZHAN1,Yi-tian DU1,Zhen-zhen YANG1,Chun-li ZHANG2,Xian-rong QI1△( )
)
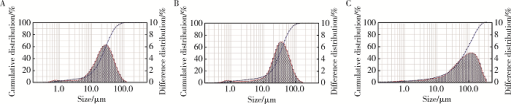
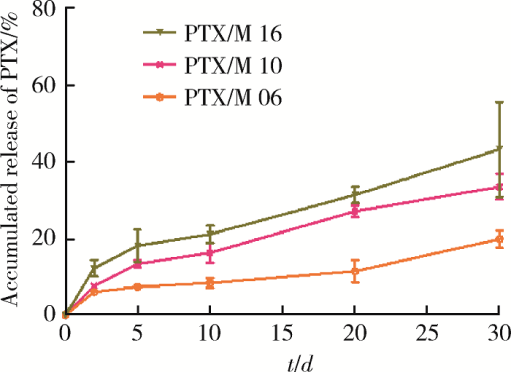
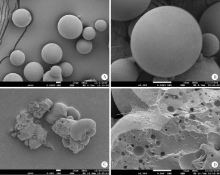
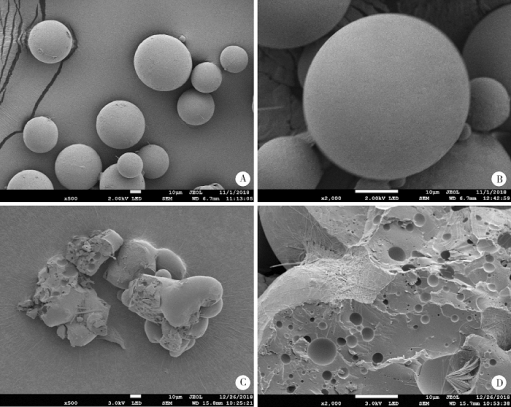
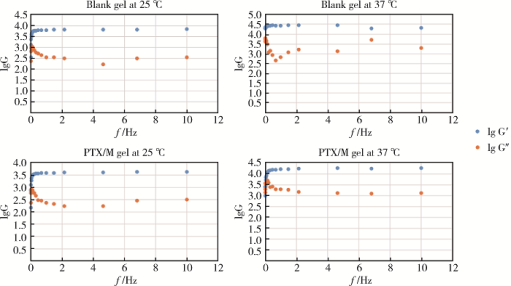
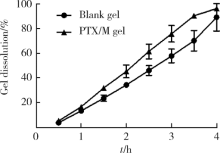
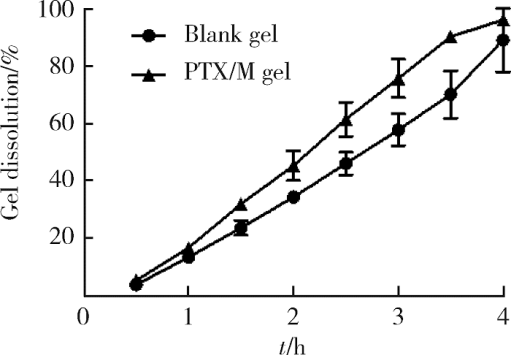
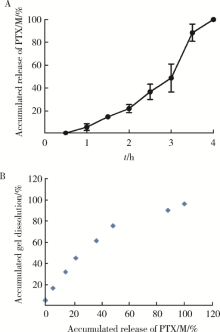
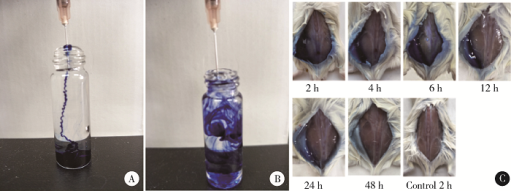
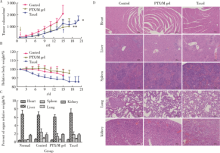
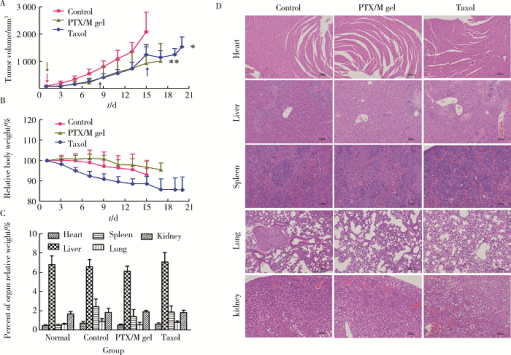
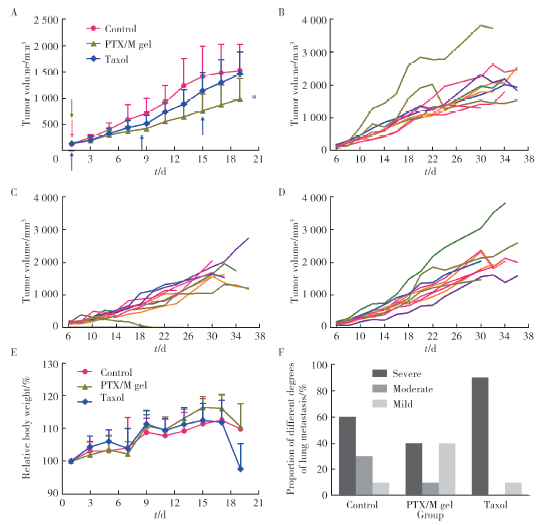
摘要: 目的 为了减少肿瘤治疗的给药次数,降低化疗药物的毒副作用,更好地控制肿瘤进展以及延缓肿瘤术后复发,制备载紫杉醇微球的温敏原位凝胶(temperature-sensitive in situ gel with paclitaxel microspheres,PTX/M gel),以术前瘤旁或术后瘤腔内局部注射的方式给药。方法 首先,通过乳化溶剂挥发法制备紫杉醇微球(paclitaxel microspheres,PTX/M),考察粒径、比表面积、形态、包封率和体外释放特性;其次,通过冷置溶解法制备PTX/M gel,测定相转变温度、弹性模量、溶蚀曲线、溶蚀-释放相关性;最后,分别建立人源U87 MG和鼠源4T1的皮下肿瘤模型,研究PTX/M gel在抑制肿瘤生长、延缓肿瘤复发方面的药效。结果 经筛选,PTX/M中位径为(32.24±1.09) μm,比表面积为(206.61±10.23) m 2/kg,包封率为85.29%±1.34%,PTX/M中紫杉醇(paclitaxel,PTX)在第30天的累积释放百分率为33.56%±3.33%;PTX/M gel相转变温度为33 ℃,在25 ℃和37 ℃时的弹性模量分别为4.2×10 3 Pa和18×10 3 Pa,体内滞留时间可达48 h。动物实验结果显示,与生理盐水组和泰素-(Taxol)组相比,PTX/M gel组小鼠的肿瘤生长最缓慢(P<0.05),体内安全性良好,同样地,在肿瘤复发实验中,PTX/M gel组小鼠术后的肿瘤复发时间最晚。 结论 PTX/M gel作为一种局部缓释制剂,能有效抑制肿瘤生长,延缓肿瘤术后复发,在肿瘤治疗中具有潜在优势。
中图分类号:
- R944.1
| [1] |
Gladson CL, Prayson RA, Liu WM . The pathobiology of glioma tumors[J]. Annu Rev Pathol, 2010,5(1):33-50.
doi: 10.1146/annurev-pathol-121808-102109 |
| [2] |
Fan L, Strasser-Weippl K, Li JJ , et al. Breast cancer in China[J]. Lancet Oncol, 2014,15(7):e279-e289.
doi: 10.1016/S1470-2045(13)70567-9 |
| [3] |
Marupudi N . Paclitaxel: a review of adverse toxicities and novel delivery strategies[J]. Expert Opin Drug Saf, 2007,6(5):609-621.
doi: 10.1517/14740338.6.5.609 |
| [4] |
Floyd JA, Galperin A, Ratner BD . Drug encapsulated polymeric microspheres for intracranial tumor therapy: A review of the literature[J]. Adv Drug Deliv Rev, 2015,91:23-37.
doi: 10.1016/j.addr.2015.04.008 |
| [5] | 顼佳音, 杨洪军, 熊欣 , 等. 常见温度敏感型原位凝胶载体的研究进展[J]. 中国实验方剂学杂志, 2011,17(2):252-257. |
| [6] |
Elkharraz K, Faisant N, Guse C , et al. Paclitaxel-loaded micro-particles and implants for the treatment of brain cancer: Preparation and physicochemical characterization[J]. Int J Pharm, 2006,314(2):127-136.
doi: 10.1016/j.ijpharm.2005.07.028 |
| [7] |
Dumortier G, Grossiord JL, Agnely F , et al. Areview of poloxamer 407 pharmaceutical and pharmacological characteristics[J]. Pharm Res, 2006,23(12):2709-2728.
doi: 10.1007/s11095-006-9104-4 |
| [8] |
Shelke S, Shahi S, Jalalpure S , et al. Formulation and evaluation of thermoreversible mucoadhesive in situ gel for intranasal delivery of Naratriptan hydrochloride[J]. J Drug Deliv Sci Technol, 2015,29:238-244.
doi: 10.1016/j.jddst.2015.08.003 |
| [9] | Pluta J, Karolewicz B . In vitro studies of the properties of thermosensitive systems prepared on Pluronic F-127 as vehicles for me-thotrexate for delivery to solid tumours[J]. Polim Med, 2006,36(3):37-53. |
| [10] |
Feng L, Qi XR, Zhou XJ , et al. Pharmaceutical and immuno-logical evaluation of a single-dose hepatitis B vaccine using PLGA microspheres[J]. J Control Release, 2006,112(1):35-42.
doi: 10.1016/j.jconrel.2006.01.012 |
| [11] |
Shive MS, Anderson JM . Biodegradation and biocompatibility of PLA and PLGA microspheres[J]. Adv Drug Deliv Rev, 1997,28(1):5-24.
doi: 10.1016/S0169-409X(97)00048-3 |
| [12] | 练有文, 王晖, 倪少凯 . BALB/c系裸小鼠脏器重量、脏器系数的测定[J]. 中国比较医学杂志, 2006,16(5):166-168. |
| [13] | Zitzmann-Kolbe S, Strube A, Frisk AL , et al. 594 in vivo detection of mammary tumor and its lung metastases in the 4T1 metastasis mouse model by PET imaging using [F-18]-D-FMT (BAY 869596)[J]. EJC Suppl, 2010,8(7):186-187. |
| [1] | 李文菁,张保宙,李恒,赖良鹏,杜辉,孙宁,龚晓峰,李莹,王岩,武勇. 胫距跟融合治疗终末期踝和后足病变的中短期临床结果[J]. 北京大学学报(医学版), 2024, 56(2): 299-306. |
| [2] | 邹雪,白小娟,张丽卿. 艾拉莫德联合托法替布治疗难治性中重度类风湿关节炎的疗效[J]. 北京大学学报(医学版), 2023, 55(6): 1013-1021. |
| [3] | 薛蔚,董樑,钱宏阳,费笑晨. 前列腺癌新辅助治疗与辅助治疗的现状及进展[J]. 北京大学学报(医学版), 2023, 55(5): 775-780. |
| [4] | 邱敏,宗有龙,王滨帅,杨斌,徐楚潇,孙争辉,陆敏,赵磊,卢剑,刘承,田晓军,马潞林. 腹腔镜肾部分切除术治疗中高复杂程度肾肿瘤的效果[J]. 北京大学学报(医学版), 2023, 55(5): 833-837. |
| [5] | 王磊,韩天栋,江卫星,李钧,张道新,田野. 主动迁移技术与原位碎石技术在输尿管软镜治疗1~2 cm输尿管上段结石中的安全性和有效性比较[J]. 北京大学学报(医学版), 2023, 55(3): 553-557. |
| [6] | 李伟浩,李伟,张学民,李清乐,焦洋,张韬,蒋京军,张小明. 去分支杂交手术和传统手术治疗胸腹主动脉瘤的结果比较[J]. 北京大学学报(医学版), 2022, 54(1): 177-181. |
| [7] | 朱正达,高岩,何汶秀,方鑫,刘洋,魏攀,闫志敏,华红. 红色诺卡氏菌细胞壁骨架治疗糜烂型口腔扁平苔藓的疗效及安全性[J]. 北京大学学报(医学版), 2021, 53(5): 964-969. |
| [8] | 李潇,苏家增,张严妍,张丽琪,张亚琼,柳登高,俞光岩. 131I相关唾液腺炎的炎症分级及内镜治疗[J]. 北京大学学报(医学版), 2020, 52(3): 586-590. |
| [9] | 陈英,刘中宁,李波,姜婷. 阿司匹林缓释微球的制备及体外缓释效果评估[J]. 北京大学学报(医学版), 2019, 51(5): 907-912. |
| [10] | 杨泽川,刘朝旭,林阳,胡伟华,陈文坚,李锋,曾恒. 颈后路单开门椎管扩大成形术全钛板与交替钛板、缝线固定治疗颈椎病的对比研究[J]. 北京大学学报(医学版), 2019, 51(1): 187-193. |
| [11] | 游文喆,窦桂丽,夏斌. 乳牙间接牙髓治疗两年疗效观察及影响因素分析[J]. 北京大学学报(医学版), 2019, 51(1): 65-69. |
| [12] | 李欣艺,赵金霞,刘湘源. 抗磷脂抗体相关性复发性流产的诊治:附75例抗磷脂综合征患者妊娠期用药和结局分析[J]. 北京大学学报(医学版), 2018, 50(6): 956-961. |
| [13] | 郭李盈,刘晓昕,李子圆,覃小雅,范则杨,李真真,关海涛,宋莉,邹英华,范田园. 空白及载阿霉素的聚丙烯酸栓塞微球的制备与评价[J]. 北京大学学报(医学版), 2018, 50(6): 1070-1077. |
| [14] | 张茗茗,郑迎东,梁宇红. 牙髓根尖周病根管治疗疗效预测模型的建立[J]. 北京大学学报(医学版), 2018, 50(1): 123-130. |
| [15] | 李旭, 李奉龙, 鲁谊, 朱以明, 郭斯翊, 李屹钧, 姜春岩. 锁定钢板治疗非骨质疏松性复杂肱骨近端骨折的中期临床及影像学随访研究[J]. 北京大学学报(医学版), 2017, 49(5): 855-860. |
|
||