北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (1): 159-166. doi: 10.19723/j.issn.1671-167X.2021.01.024
低氧状态及炎症反应是新型冠状病毒肺炎患者发生急性心肌损伤的危险因素
杨林承1,张瑞涛1,郭丽君1,肖晗1,祖凌云1,张幼怡1,程秦2,赵志伶3,葛庆岗3,高炜1,Δ( )
)
- 1.北京大学第三医院心内科,国家卫生健康委员会心血管分子生物学与调节肽重点实验室,分子心血管学教育部重点实验室,心血管受体研究北京市重点实验室,北京 100191
2.北京大学第三医院呼吸与危重医学科,北京 100191
3.北京大学第三医院危重医学科,北京 100191
Hypoxia and inflammation are risk factors for acute myocardial injury in patients with coronavirus disease 2019
YANG Lin-cheng1,ZHANG Rui-tao1,GUO Li-jun1,XIAO Han1,ZU Ling-yun1,ZHANG You-yi1,CHENG Qin2,ZHAO Zhi-ling3,GE Qing-gang3,GAO Wei1,Δ( )
)
- 1. Department of Cardiology and Institute of Vascular Medicine, Peking University Third Hospital & NHC Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides & Key Laboratory of Molecular Cardiovascular Science, Ministry of Education & Beijing Key Laboratory of Cardiovascular Receptors Research, Beijing 100191, China
2. Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing 100191, China
3. Department of Intensive Care Medicine, Peking University Third Hospital, Beijing 100191, China
摘要:
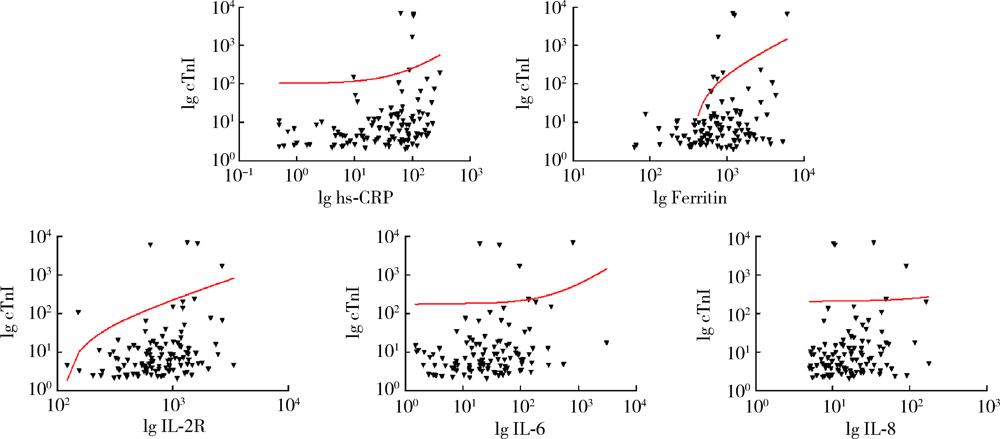
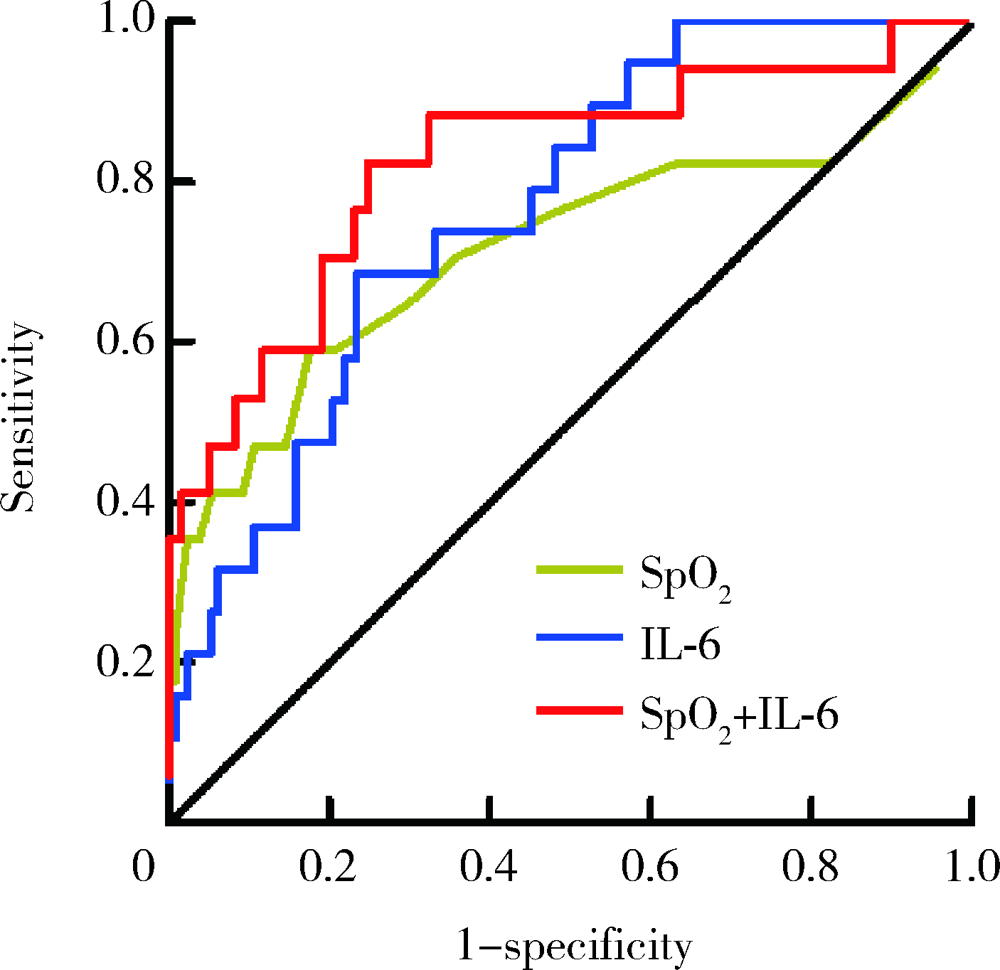
目的: 寻找新型冠状病毒肺炎(coronavirus disease 2019,COVID-19)患者发生急性心肌损伤的危险因素。方法: 本研究为单中心COVID-19住院患者的回顾性队列研究,共纳入149例COVID-19确诊患者,依据2018年欧洲心脏病学会年会发布的第四版心肌梗死全球统一定义中关于心肌损伤的诊断标准,将患者分为心肌损伤组(19例)及非心肌损伤组(130例)。收集全部入组患者的临床信息及实验室化验结果,并分析COVID-19患者发生急性心肌损伤的危险因素。结果: 与非心肌损伤组患者相比,心肌损伤组患者年龄更大,危重症患者比例更高(P<0.05),入院时呼吸频率更快,未吸氧状态外周血氧饱和度(percutaneous oxygen saturation,SpO2)偏低(P<0.05)。心肌损伤组患者除肿瘤坏死因子α(tumor necrosis factor α,TNF-α)外,其余炎症因子水平均高于非心肌损伤组(P<0.05)。多因素Logistic回归分析发现,入院时未吸氧状态SpO2水平偏低(OR=0.860,95%CI:0.779~0.949,P=0.003)及血白细胞介素-6(interleukin-6,IL-6)水平升高(OR=1.068,95%CI:1.019~1.120,P=0.006)为COVID-19患者发生急性心肌损伤的独立危险因素。结论: 低氧状态及炎症在COVID-19患者发生急性心肌损伤的病理生理过程中起到了重要作用。
中图分类号:
- R542.2
| [1] |
Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission[J]. Sci China Life Sci, 2020,63(3):457-460.
doi: 10.1007/s11427-020-1637-5 pmid: 32009228 |
| [2] |
Lu R, Zhao X, Li J, et al. Genomic characterisation and epide-miology of 2019 novel coronavirus: Implications for virus origins and receptor binding[J]. Lancet, 2020,395(10224):565-574.
doi: 10.1016/S0140-6736(20)30251-8 pmid: 32007145 |
| [3] |
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study[J]. Lancet, 2020,395(10223):507-513.
doi: 10.1016/S0140-6736(20)30211-7 pmid: 32007143 |
| [4] |
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020,395(10223):497-506.
doi: 10.1016/S0140-6736(20)30183-5 pmid: 31986264 |
| [5] |
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China[J]. JAMA, 2020,323(11):1061-1069.
doi: 10.1001/jama.2020.1585 pmid: 32031570 |
| [6] | 魏之瑶, 钱海燕. 新型冠状病毒肺炎患者的心肌损伤表现[J]. 中华心血管病杂志, 2020,48(6):439-442. |
| [7] | Fourth universal definition of myocardial infarction (2018)[J]. Rev Esp Cardiol (Engl Ed), 2019,72(1):72. |
| [8] | 陈晨, 陈琛, 严江涛, 等. 新型冠状病毒肺炎危重型患者心肌损伤及患有心血管基础疾病的情况分析[J]. 中华心血管病杂志, 2020,48(7):567-571. |
| [9] |
Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China[J]. N Engl J Med, 2020,382(18):1708-1720.
doi: 10.1056/NEJMoa2002032 pmid: 32109013 |
| [10] | Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues[J]. Cancer Res, 1994,54(6):1425-1430. |
| [11] | Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction[J]. Am J Physiol Cell Physiol, 2001,280(4):C719-C741. |
| [12] | Csiszar A, Wang M, Lakatta EG, et al. Inflammation and endothelial dysfunction during aging: role of NF-kappaB[J]. J Appl Physiol (1985), 2008,105(4):1333-1341. |
| [13] | Iida T, Mine S, Fujimoto H, et al. Hypoxia-inducible factor-1alpha induces cell cycle arrest of endothelial cells[J]. Genes Cells, 2002,7(2):143-149. |
| [14] | Aoki M, Nata T, Morishita R, et al. Endothelial apoptosis induced by oxidative stress through activation of NF-kappaB: antiapoptotic effect of antioxidant agents on endothelial cells[J]. Hypertension, 2001,38(1):48-55. |
| [15] | Chen Y, Liu Y, Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis[J]. Circ Res, 2011,109(12):1327-1331. |
| [16] | Chen Q, Moghaddas S, Hoppel CL, et al. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria[J]. Am J Physiol Cell Physiol, 2008,294(2):C460-C466. |
| [17] | Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: Mechanisms and translational implications[J]. Cell Death Dis, 2011,2(12):e244. |
| [18] | Nunez C, Victor VM, Marti M, et al. Role of endothelial nitric oxide in pulmonary and systemic arteries during hypoxia[J]. Nitric Oxide, 2014,37:17-27. |
| [19] | Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study[J]. Lancet Respir Med, 2020,8(5):475-481. |
| [20] | Hui H, Zhang YQ, Yang X, et al. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia[J]. MedRxiv, 2020, 2020.02.24.20027052. |
| [21] |
Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology[J]. Semin Immunopathol, 2017,39(5):529-539.
doi: 10.1007/s00281-017-0629-x pmid: 28466096 |
| [22] | Neumann FJ, Ott I, Marx N, et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity[J]. Arterioscler Thromb Vasc Biol, 1997,17(12):3399-3405. |
| [23] |
Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm[J]. Immunotherapy, 2016,8(8):959-970.
doi: 10.2217/imt-2016-0020 pmid: 27381687 |
| [24] |
Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China[J]. J Pathol, 2003,200(3):282-289.
pmid: 12845623 |
| [25] | 国家卫生健康委员会. 新型冠状病毒诊疗方案(试行第七版)[EB]. (2020-03-03)[2020-04-15]. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. |
| [26] |
Watts ER, Walmsley SR. Inflammation and hypoxia: HIF and PHD isoform selectivity[J]. Trends Mol Med, 2019,25(1):33-46.
doi: 10.1016/j.molmed.2018.10.006 pmid: 30442494 |
| [27] | Campbell EL, Bruyninckx WJ, Kelly CJ, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation[J]. Immunity, 2014,40(1):66-77. |
| [1] | 马会超,李军,王永清. 妊娠合并炎症性肠病的临床特点[J]. 北京大学学报(医学版), 2024, 56(2): 260-266. |
| [2] | 俞光岩. 儿童唾液腺疾病[J]. 北京大学学报(医学版), 2024, 56(1): 1-3. |
| [3] | 殳畅,韩烨,孙雨哲,杨再目,侯建霞. Ⅲ期牙周炎患者牙周基础治疗前后炎症性贫血相关指标的变化[J]. 北京大学学报(医学版), 2024, 56(1): 45-50. |
| [4] | 梁秀睿,闪雪纯,关晶,张锐,杨静,张怡,金佳琦,张誉馨,徐凡,傅继华. 高血糖诱导肝星状细胞5-羟色胺降解在2型糖尿病致肝脏炎症和纤维化时的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1141-1150. |
| [5] | 贺冰洁,刘志科,沈鹏,孙烨祥,陈彬,詹思延,林鸿波. 2011—2020年宁波市鄞州区炎症性肠病发病的流行病学研究[J]. 北京大学学报(医学版), 2022, 54(3): 511-519. |
| [6] | 郭辅政,赵秀娟,邓玖旭,杜哲,王天兵,朱凤雪. 严重创伤患者早期外周血淋巴细胞变化与预后之间的关系[J]. 北京大学学报(医学版), 2022, 54(3): 552-556. |
| [7] | 王向熙,李臻臻,赖彦云,杨莉,史霖丽,仲少敏,吴艳. 585 nm Q开关激光治疗痤疮炎症性皮损和炎症后红斑的疗效[J]. 北京大学学报(医学版), 2022, 54(2): 283-288. |
| [8] | 伊文霞,魏翠洁,吴晔,包新华,熊晖,常杏芝. 长疗程利妥昔单抗治疗难治性幼年型特发性炎症性肌病3例[J]. 北京大学学报(医学版), 2021, 53(6): 1191-1195. |
| [9] | 陈怀安,刘硕,李秀君,王哲,张潮,李凤岐,苗文隆. 炎症生物标志物对输尿管尿路上皮癌患者预后预测的临床价值[J]. 北京大学学报(医学版), 2021, 53(2): 302-307. |
| [10] | 胡永玮,刘蕊,罗莉. 慢性多灶性骨髓炎1例及文献回顾[J]. 北京大学学报(医学版), 2020, 52(6): 1140-1145. |
| [11] | 轩艳,蔡宇,王啸轩,石巧,邱立新,栾庆先. 牙龈卟啉单胞菌感染对载脂蛋白e基因敲除小鼠动脉粥样硬化的影响[J]. 北京大学学报(医学版), 2020, 52(4): 743-749. |
| [12] | 李军,牛占岳,薛艳,石雪迎,张波,王媛. 重度溃疡性结肠炎合并卡波西肉瘤1例并文献综述[J]. 北京大学学报(医学版), 2020, 52(2): 373-377. |
| [13] | 王平,宋婧,方翔宇,李鑫,刘栩,贾园,栗占国,胡凡磊. 成红细胞样Ter细胞在胶原诱导性关节炎发病中的作用[J]. 北京大学学报(医学版), 2019, 51(3): 445-450. |
| [14] | 段丽萍,郑朝霞,张宇慧,董捷. 腹膜透析患者营养不良-炎症-心血管疾病与认知功能恶化的关系[J]. 北京大学学报(医学版), 2019, 51(3): 510-518. |
| [15] | 刘佳兴,胡贵平,赵琳,张永明,王丽,贾光,刘瑞祥,冯慧敏,徐华东. 铬酸盐低水平长期职业接触与劳动者早期健康效应[J]. 北京大学学报(医学版), 2019, 51(2): 307-314. |
|
||