北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (2): 378-383. doi: 10.19723/j.issn.1671-167X.2021.02.024
掺锶磷酸钙骨水泥材料生物学性能的动物实验
- 1.北京大学第三医院口腔科, 北京 100191
2.海南医学院第二附属医院口腔科, 海口 570311
In vivo study of strontium-doped calcium phosphate cement for biological properties
WANG Jing-qi1,2,WANG Xiao1,Δ( )
)
- 1. Department of Stomatology, Peking University Third Hospital, Beijing 100191, China
2. Department of Stomatology, the Second Affiliated Hospital of Hainan Medical University, Haikou 570311, China
摘要:
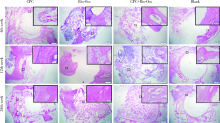
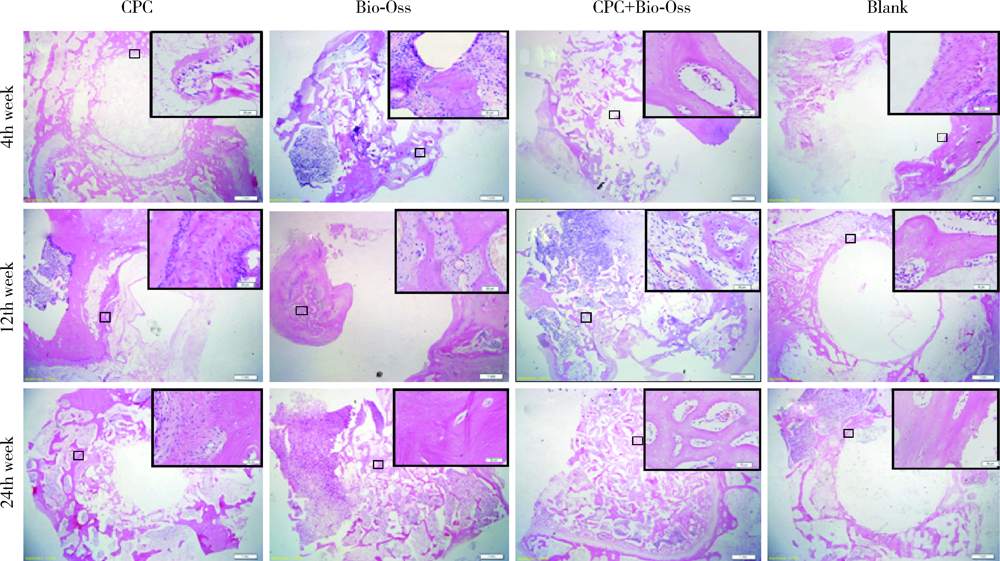
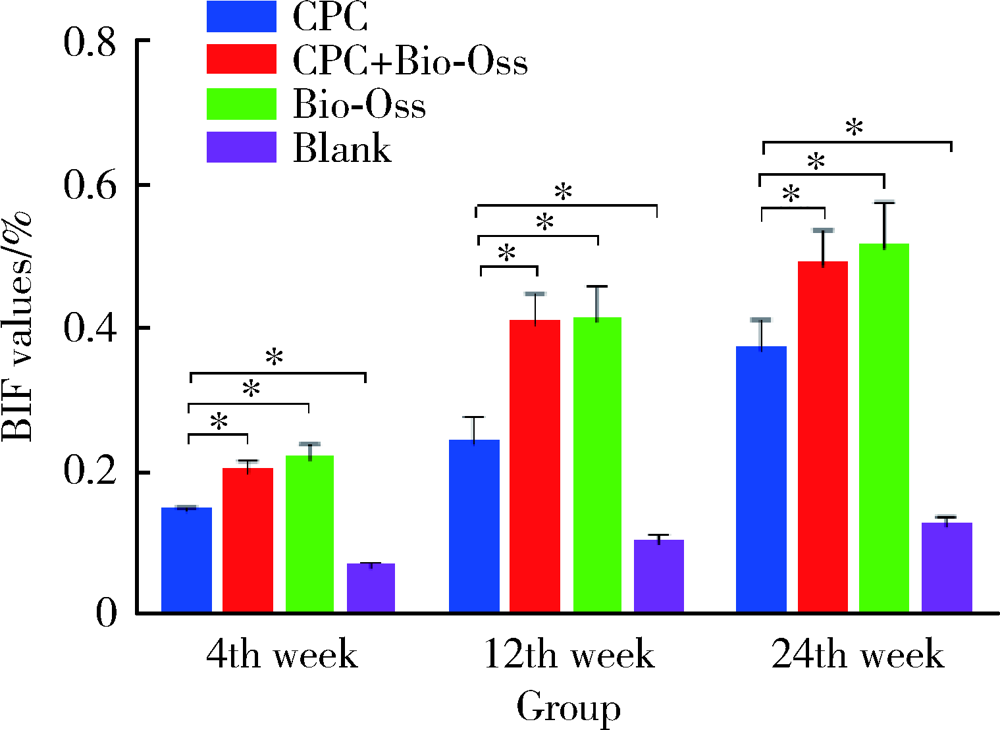
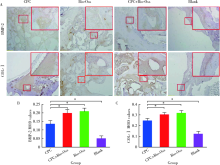
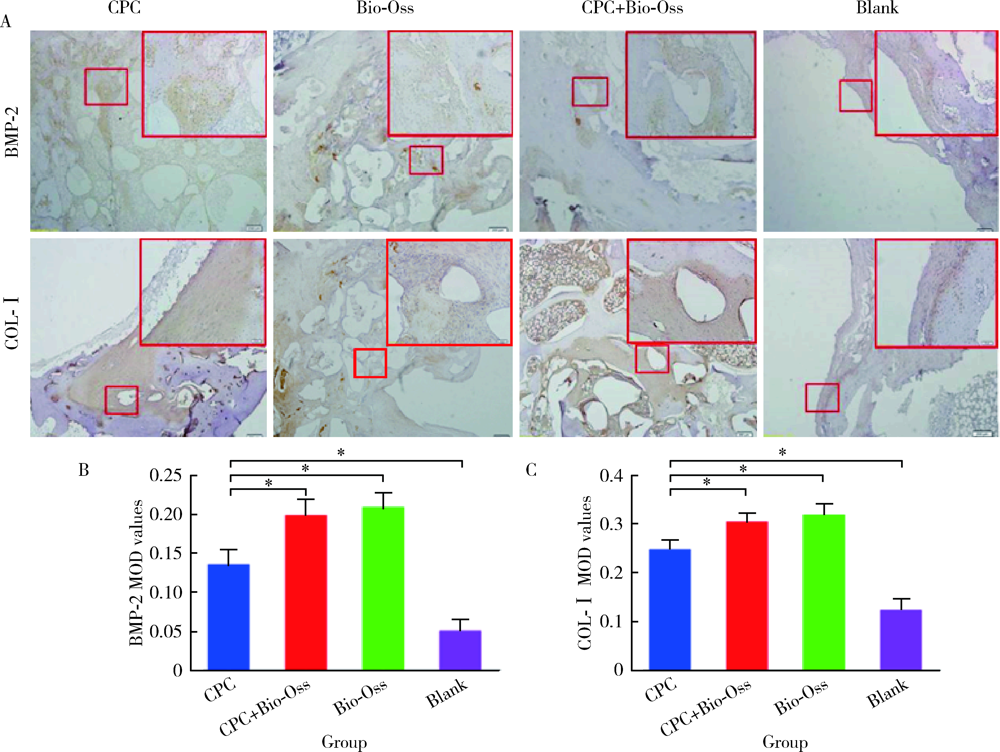
目的: 评价新型磷酸钙骨水泥(calcium phosphate cement,CPC)的生物相容性以及成骨效果,为其进一步的临床应用提供实验数据。方法: 选择新西兰大白兔30只,以其双后腿外侧髁(60个)为实验对象,随机分为CPC组、CPC+Bio-Oss组、Bio-Oss组和空白对照组4组,在兔双侧后腿外侧髁制造直径6 mm、深7 mm的骨缺损模型,按照组别分别植入CPC、Bio-Oss、CPC+Bio-Oss混合物(CPC与Bio-Oss骨粉质量比为4 ∶1)。实验动物分别在手术第4周、第12周、第24周处死,取骨缺损周围组织,HE染色进行组织学评价,并计算新骨生成率(bone ingrowth fraction,BIF); 利用免疫组织化学染色,计算阳性区域平均光密度(mean optical density,MOD), 检测术后第4周各组样本BMP-2和COL-Ⅰ的表达情况,评价各组样本在不同时间点的骨愈合情况。结果: HE染色发现,在相同时间点,与空白对照组相比, CPC组、CPC+Bio-Oss组、Bio-Oss组的BIF值明显较高(P<0.01), 其中,CPC组BIF低于Bio-Oss组和CPC+Bio-Oss组(P<0.01),CPC+Bio-Oss组与Bio-Oss组相比差异无统计学意义(P>0.05)。免疫组织化学染色结果显示,与空白对照组相比,CPC组BMP-2和COL-Ⅰ的MOD值较高,但低于Bio-Oss组和CPC+Bio-Oss组(P<0.01), CPC+Bio-Oss组BMP-2和COL-Ⅰ的MOD与Bio-Oss组相比差异无统计学意义(P>0.05)。结论: 新型磷酸钙骨水泥具有良好的生物相容性,可以促进早期成骨,成骨效果稳定,长期有效。
中图分类号:
- R783.1
| [1] |
Choudhary S, Halbout P, Alander C, et al. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins[J]. J Bone Miner Res, 2007,22(7):1002-1010.
pmid: 17371157 |
| [2] | Baier M, Staudt P, Klein R. Strontium enhances osseointegration of calcium phosphate cement: a histomorphometric pilot study in ovariectomized rats[J]. J Orthop Surg Res, 2013,8(1):16. |
| [3] | 李曙霞, 王京旗. 掺锶磷酸钙骨水泥材料的生物学性能体外研究[J]. 北京口腔医学, 2017,25(2):81-84. |
| [4] | 王晓娜, 赵静辉. 骨替代材料在口腔种植领域中的成骨效果[J]. 国际口腔医学杂志, 2016,43(1):113-117. |
| [5] |
Meloni SM, Jovanovic SA, Lolli FM, et al. Grafting after sinus lift with anorganic bovine bone alone compared with 50 ∶50 anorganic bovine bone and autologous bone: results of a pilot randomised trial at one year[J]. Br J Oral Maxillofac Surg, 2015,53(5):436-441.
pmid: 25796408 |
| [6] |
Aludden HC, Mordenfeld A, Hallman M, et al. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: a systematic review[J]. Int J Oral Maxillofac Surg, 2017,46(8):1030-1038.
doi: 10.1016/j.ijom.2017.03.008 pmid: 28366452 |
| [7] | Wang F, Li Q, Wang Z. A comparative study of the effect of Bio-Oss in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting[J]. J Oral Patho Med, 2017,46(7):528-536. |
| [8] | Orsini G, Scarano A, Degidi M, et al. Histological and ultrastructural evaluation of bone around Bio-Oss particles in sinus augmentation[J]. Oral Dis, 2007,13(13):586-593. |
| [9] | Jensen T, Schou S, Stavropoulos A, et al. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: a systematic review[J]. Int J Oral Maxillofac Surg, 2012,41(1):114-120. |
| [10] |
Urban IA, Nagursky H, Lozada JL, et al. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 25 patients[J]. Int J Periodontics Restorative Dent, 2013,33(3):299-307.
pmid: 23593623 |
| [11] | Tian M, Chen F, Song W. In vivo study of porous strontium-doped calcium poly-phosphate scaffolds for bone substitute applications[J]. J Mater Sci Mater Med, 2009,20(7):1505-1512. |
| [12] | 严广斌. 骨组织形态计量学[J]. 中华关节外科杂志: 电子版, 2016,10(2):100. |
| [13] | 郭冲冲, 杜启翠. 细胞因子与牙槽骨重建关系的研究进展[J]. 口腔医学, 2015,35(8):697-701. |
| [14] | Axelrad TW, Kakar S, Einhorn TA. New technologies for the enhancement of skeletal repair[J]. Injury, 2007,38(l1):49-62. |
| [15] | 苏佳灿. 骨生长因子[M]. 第二军医大学出版社, 2015: 170-182. |
| [16] |
Marelli B, Ghezzi CE, Barralet JE, et al. Three-dimensional mineralization of dense nanofibrillar collagen bioglass hybrid scaffolds[J]. Biomacromolecules, 2010,11(6):1470-1479.
pmid: 20443577 |
| [17] |
Thorwarth M, Rupprecht S, Falk S, et al. Expression of bone matrix proteins during de novo bone formation using a bovine collagen and platelet-rich plasma (prp): an immunohistochemical analysis[J]. Biomaterials, 2005,26(15):2575-2584.
pmid: 15585260 |
| [18] | 郭莉. CPC与人重组骨形成蛋白-2(rhBMP-2)复合修复即刻种植牙骨缺损的效果研究[J]. 现代仪器与医疗, 2015,6(6):104-106. |
| [19] | Dahl SG, Allain P, Marie PJ, et al. Incorporation and distribution of strontium in bone[J]. Bone (New York), 2001,28(4):446-453. |
| [1] | 黄莹,吴志远,周行红,蔡志刚,张杰. 股前外侧皮瓣修复上颌骨缺损术后面部软组织对称性感观分级[J]. 北京大学学报(医学版), 2023, 55(4): 708-715. |
| [2] | 康一帆,单小峰,张雷,蔡志刚. 游离腓骨瓣修复重建上颌骨术后腓骨瓣位置变化[J]. 北京大学学报(医学版), 2020, 52(5): 938-942. |
| [3] | 吴唯伊,李博文,刘玉华,王新知. 复层猪小肠黏膜下层可吸收膜的降解性能[J]. 北京大学学报(医学版), 2020, 52(3): 564-569. |
| [4] | 曹畅,王菲,王恩博,刘宇. β-磷酸三钙用于下颌第三磨牙拔除术后骨缺损修复的自身对照研究[J]. 北京大学学报(医学版), 2020, 52(1): 97-102. |
| [5] | 李博文,吴唯伊,唐琳,张一,刘玉华. 改良猪小肠黏膜下层可吸收膜在兔下颌骨缺损早期愈合中的作用[J]. 北京大学学报(医学版), 2019, 51(5): 887-892. |
| [6] | 梁晨,张维宇,胡浩,王起,方志伟,许克新. 膀胱扩大术两种不同术式的疗效及并发症比较[J]. 北京大学学报(医学版), 2019, 51(2): 293-297. |
| [7] | 詹雅琳,胡文杰,徐涛,甄敏,路瑞芳. 罹患重度牙周炎磨牙拔除后应用去蛋白牛骨基质与可吸收胶原膜进行位点保存的组织学研究[J]. 北京大学学报(医学版), 2017, 49(1): 169-175. |
| [8] | 章文博,于尧,王洋,刘筱菁,毛驰,郭传瑸,俞光岩,彭歆. 数字化外科技术在上颌骨缺损重建中的应用[J]. 北京大学学报(医学版), 2017, 49(1): 1-005. |
| [9] | 宋杨,王晓飞,王宇光,孙玉春,吕培军△. 人脂肪间充质干细胞与生物材料共混物三维打印体的体内成骨[J]. 北京大学学报(医学版), 2016, 48(1): 45-50. |
| [10] | 詹雅琳, 胡文杰, 甄敏, 徐涛, 路瑞芳. 去蛋白牛骨基质与可吸收胶原膜的磨牙拔牙位点保存效果影像学评价[J]. 北京大学学报(医学版), 2015, 47(1): 19-26. |
| [11] | 张培训,安帅,王国强,王艳华,陈博,王振威,韩娜,寇玉辉,王韵,姜保国. 生物套管小间隙套接修复大鼠坐骨神经损伤过程中的疼痛评估[J]. 北京大学学报(医学版), 2013, 45(5): 675-678. |
| [12] | 张培训, 寇玉辉, 韩娜, 党育, 薛峰, 王天兵, 徐海林, 陈建海, 杨明, 卢浩, 殷晓峰, . 可降解生物套管小间隙套接法修复周围神经损伤的临床观察[J]. 北京大学学报(医学版), 2012, 44(6): 842-846. |
| [13] | 米姗姗, 董艳梅, 高学军. 溶胶-凝胶生物活性玻璃对人牙髓细胞作用的研究[J]. 北京大学学报(医学版), 2012, 44(1): 39-42. |
| [14] | 任晓帅, 魏世成, , 苏晓东. 牙种植钛基生物材料表面骨形态发生蛋白修饰的固定与评价方法[J]. 北京大学学报(医学版), 2010, 42(5): 604-607. |
|
||