北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (5): 895-902. doi: 10.19723/j.issn.1671-167X.2025.05.013
溃疡性结肠炎患者唾液外泌体内蛋白标志物的筛选及功能分析
杨丛艺1, 郑小雯2, 陈静宜1, 徐俊1, 陈峰2, 陈扬3, 陈宁1,*( )
)
- 1. 北京大学人民医院消化内科,北京 100044
2. 北京大学口腔医学院·口腔医院中心实验室,国家口腔医学中心,国家口腔疾病临床医学研究中心,口腔生物材料和数字诊疗装备国家工程研究中心,北京 100081
3. 北京大学基础医学院精准医疗多组学研究中心,北京 100191
Protein biomarker screening and functional analysis of salivary exosomes in patients with ulcerative colitis
Congyi YANG1, Xiaowen ZHENG2, Jingyi CHEN1, Jun XU1, Feng CHEN2, Yang CHEN3, Ning CHEN1,*( )
)
- 1. Department of Gastroenterology, Peking University People's Hospital, Beijing 100044, China
2. Central Laboratory, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
3. Center for Precision Medicine Multi-Omics Research, Peking University School of Basic Medical Science, Beijing 100191, China
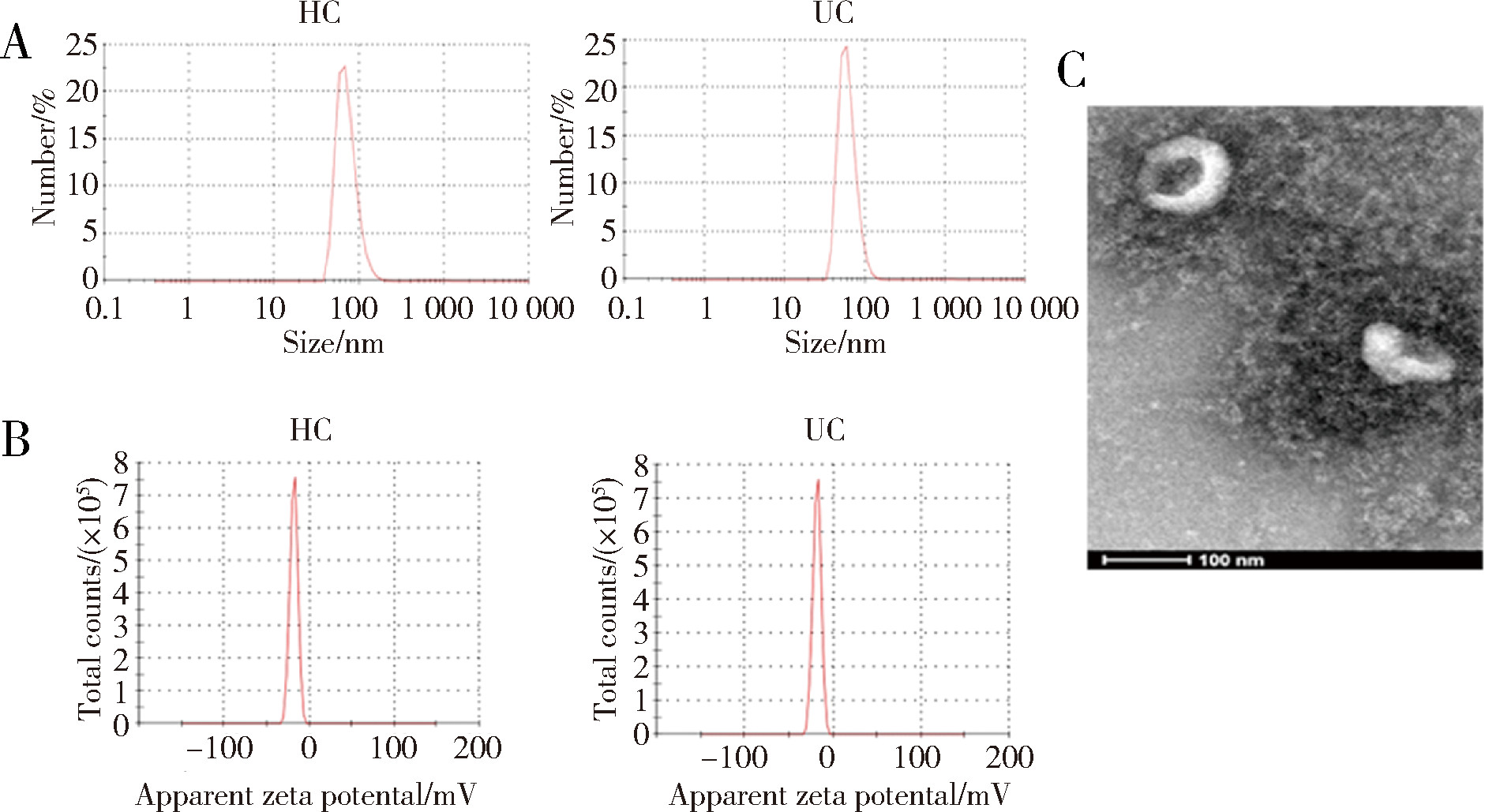
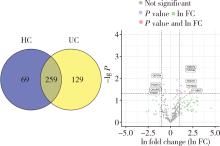
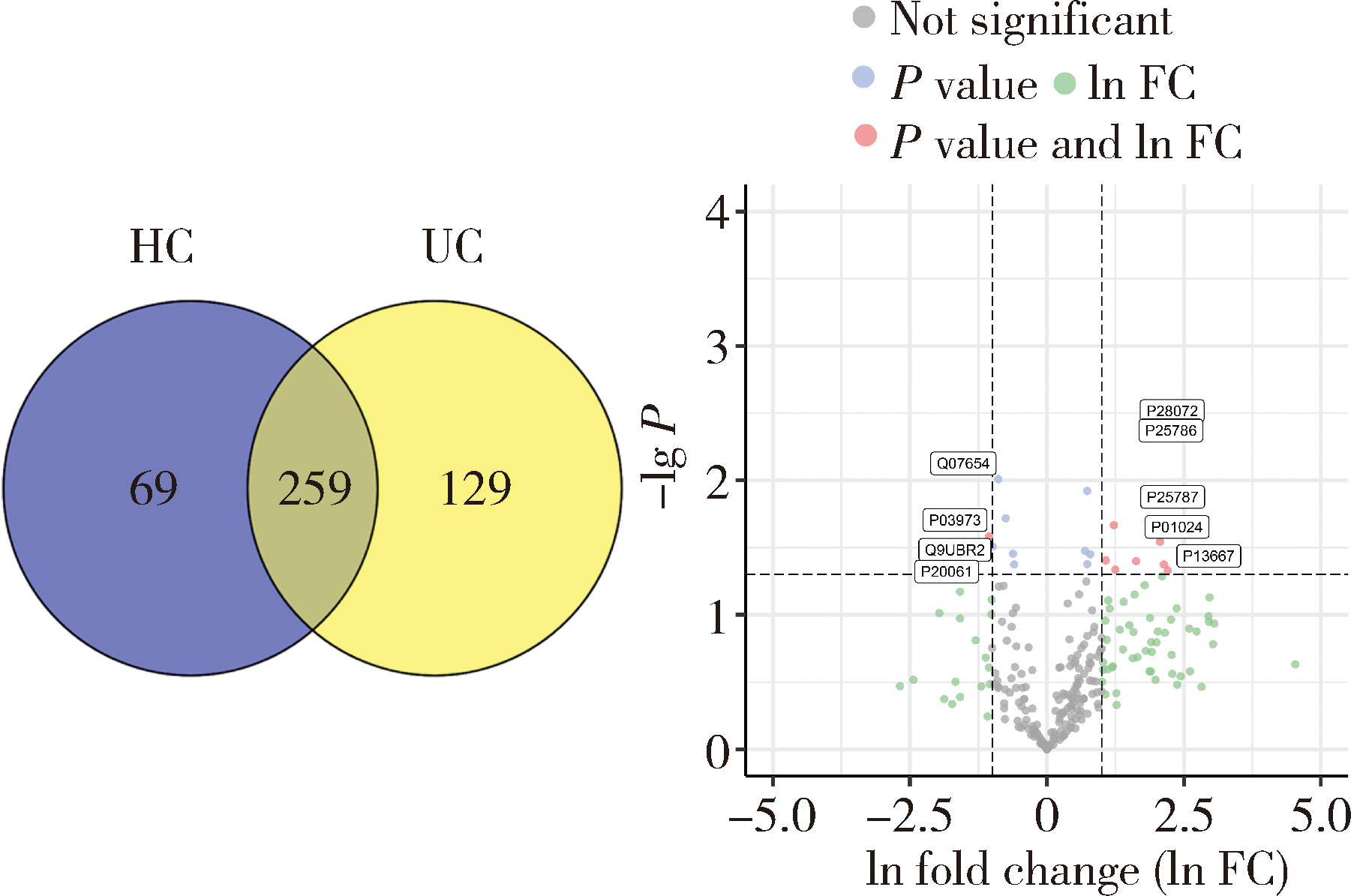
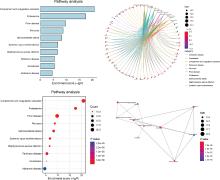
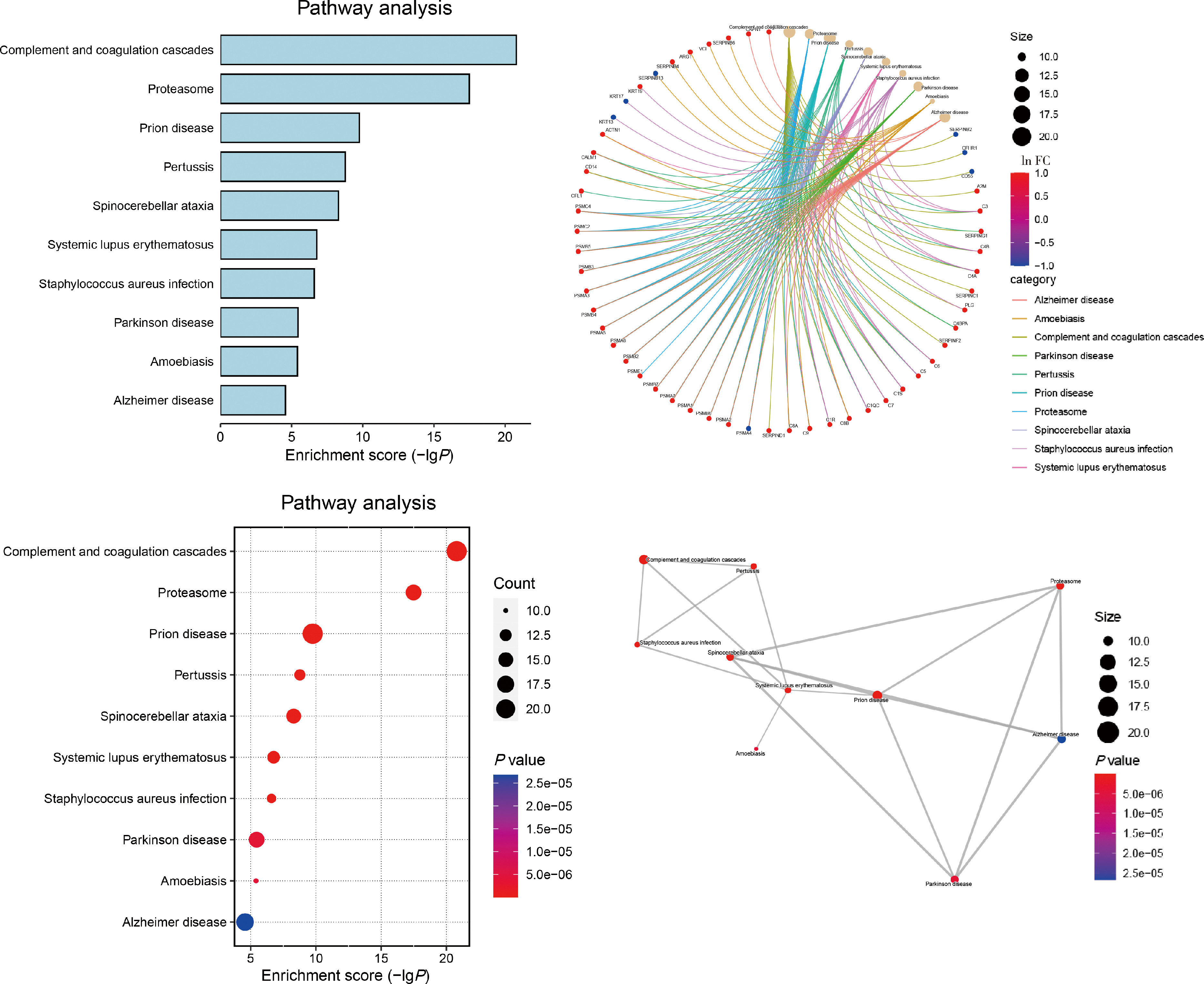
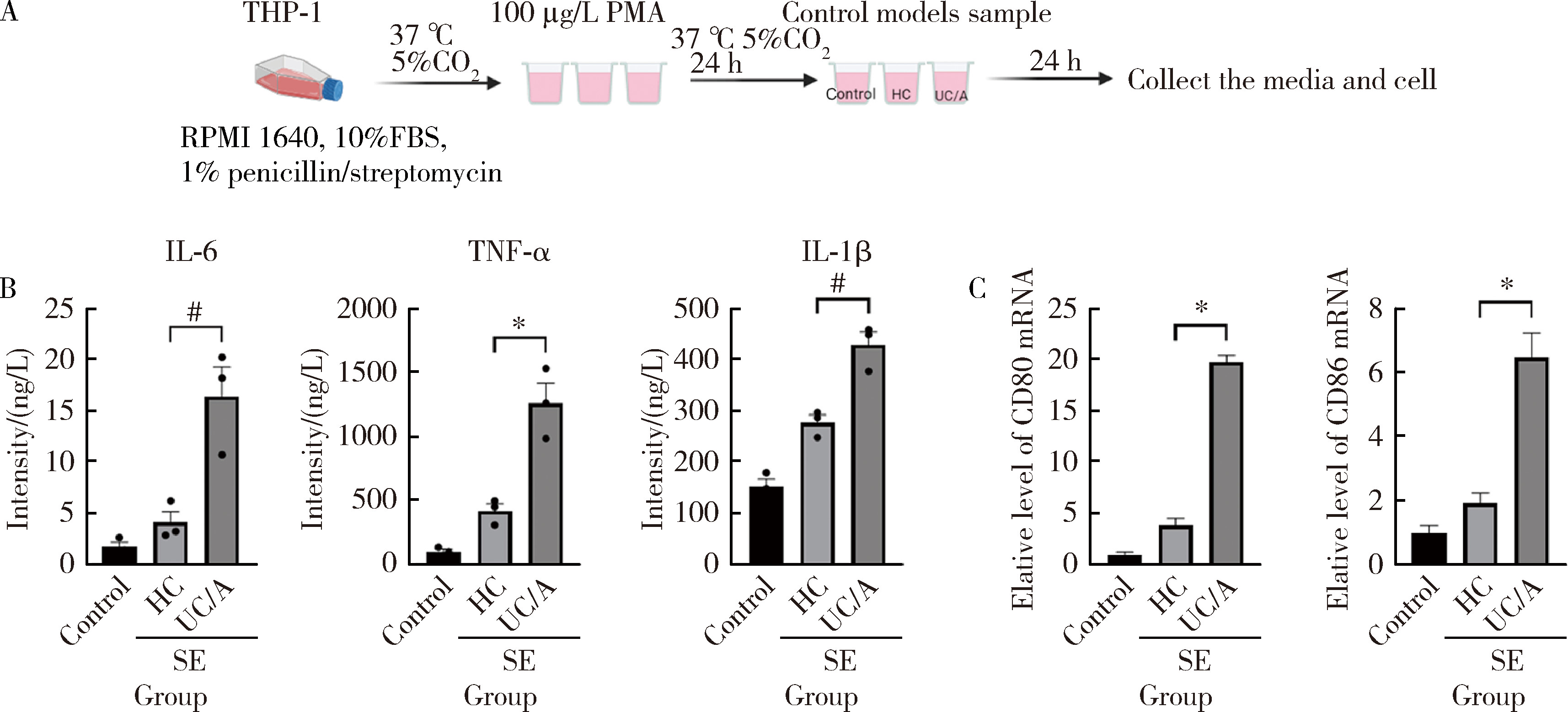
摘要: 目的: 筛选可能与溃疡性结肠炎(ulcerative colitis,UC)密切相关的蛋白标志物, 探索UC患者唾液外泌体特异性高表达蛋白的功能及在UC发病中的作用。方法: 2021年7月至2022年6月从北京大学人民医院消化内科共招募初诊初治活动期UC患者37例,健康对照(healthy control,HC) 志愿者10人,收集全部受试者唾液后分别提取唾液外泌体,用于Shotgun质谱分析及后续实验,寻找UC组和HC组之间差异表达的蛋白质。利用DAVID工具对差异表达蛋白的基因进行GO(gene ontology)、KEGG(Kyoto encyclopedia of genes and genomes)富集分析。体外实验将UC组与HC组唾液外泌体分别与巨噬细胞共培养,实时定量聚合酶链反应(real-time quantitative PCR,qPCR)检测细胞CD80+、CD86+水平;ELISA法检测细胞上清液白介素-6(interleukin-6,IL-6)、白介素-1β(interleukin-1β,IL-1β)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)分泌水平。结果: UC组与HC组唾液外泌体中共表达的蛋白质有259种,其中11种蛋白质在UC组中高表达,包括PDIA4、A2M、EEF2、C3、PSMA2、PSMB6、PSMA1、IGHG1、IGHG3、IGHG4、SERPING1,4种蛋白质在UC组中低表达,包括TCN1、SLPI、CTSZ、TFF3,将这15种蛋白质与仅存在于UC患者中的129种特异蛋白质,以及仅存在于HC组中的69种特异蛋白质共同进行GO/KEGG功能学分析,发现UC组唾液外泌体中蛋白酶体相关蛋白PSMA1、PSMA2、PSMB6表达升高,以及补体级联通路中多种关键分子(如C3等)表达上调。体外共培养实验发现,与HC组相比,活动期UC患者唾液外泌体可以通过促进巨噬细胞向M1型转化并分泌炎性因子IL-1β、IL-6、TNF-α发挥促炎作用。结论: UC患者唾液外泌体可能具有促进炎症的功能,对UC患者及健康对照志愿者唾液外泌体内蛋白质进行分析,发现在两组共表达的蛋白质中有15种蛋白质表达量存在明显差异,其中UC组高表达的C3、PSMA2、PSMB6、PSMA1主要与免疫及炎症反应相关,提示UC患者唾液外泌体中特异性高表达的蛋白质有作为UC诊断疾病标志物的潜力,并可能在UC的发病中发挥作用。
中图分类号:
- R574.62
| 1 |
|
| 2 |
doi: 10.1016/S0140-6736(17)32448-0 |
| 3 |
doi: 10.1038/nature06005 |
| 4 |
doi: 10.1136/gut.2005.069476 |
| 5 |
doi: 10.1126/science.aau6977 |
| 6 |
doi: 10.1073/pnas.1618088114 |
| 7 |
doi: 10.1002/adma.201802896 |
| 8 |
doi: 10.1089/omi.2011.0118 |
| 9 |
doi: 10.1093/bioinformatics/btt285 |
| 10 |
doi: 10.3390/cells8070727 |
| 11 |
doi: 10.1111/prd.12100 |
| 12 |
doi: 10.1016/j.arr.2022.101587 |
| 13 |
doi: 10.7150/ijbs.25018 |
| 14 |
|
| 15 |
doi: 10.1186/s11658-021-00280-x |
| 16 |
doi: 10.1016/j.stem.2012.11.009 |
| 17 |
doi: 10.1136/gutjnl-2012-303205 |
| 18 |
doi: 10.1186/s41232-016-0011-8 |
| 19 |
doi: 10.1038/nm.1978 |
| 20 |
doi: 10.1016/j.addr.2011.05.010 |
| 21 |
doi: 10.1002/pmic.201500174 |
| 22 |
doi: 10.1136/gut.30.3.361 |
| 23 |
doi: 10.1126/science.aan4526 |
| 24 |
doi: 10.1097/MIB.0000000000000840 |
| [1] | 曹芳,钟明,刘从容. 宫体POLE突变型内膜样癌合并HPV感染相关性宫颈腺癌1例报道及文献回顾[J]. 北京大学学报(医学版), 2023, 55(2): 370-374. |
| [2] | 梁丽,李鑫,农琳,董颖,张继新,李东,李挺. 子宫内膜癌微卫星不稳定性分析: 微小微卫星变换的意义[J]. 北京大学学报(医学版), 2023, 55(2): 254-261. |
| [3] | 张波. 弥漫性神经内分泌细胞肿瘤病理学:共性与异质性[J]. 北京大学学报(医学版), 2023, 55(2): 210-216. |
| [4] | 柯杨,王敏敏,刘萌飞,刘芳芳,刘英,何忠虎. 肿瘤早期预警生物标志物的研究与思考[J]. 北京大学学报(医学版), 2022, 54(5): 810-813. |
| [5] | 蔡天玉,朱振鹏,徐纯如,吉星,吕同德,郭振可,林健. 成纤维细胞生长因子受体2在肾透明细胞癌中的表达及意义[J]. 北京大学学报(医学版), 2022, 54(4): 628-635. |
| [6] | 贺冰洁,刘志科,沈鹏,孙烨祥,陈彬,詹思延,林鸿波. 2011—2020年宁波市鄞州区炎症性肠病发病的流行病学研究[J]. 北京大学学报(医学版), 2022, 54(3): 511-519. |
| [7] | 陈怀安,刘硕,李秀君,王哲,张潮,李凤岐,苗文隆. 炎症生物标志物对输尿管尿路上皮癌患者预后预测的临床价值[J]. 北京大学学报(医学版), 2021, 53(2): 302-307. |
| [8] | 王婷婷,韩影,高芳芳,叶磊,张育军. 环状RNA circ-SOD2对肠上皮屏障和溃疡性结肠炎的作用[J]. 北京大学学报(医学版), 2019, 51(5): 805-812. |
| [9] | 杨飞龙,洪锴,赵国江,刘承,宋一萌,马潞林. 基于长链非编码RNA的生物信息学分析构建膀胱癌预后模型并确定预后生物标志物[J]. 北京大学学报(医学版), 2019, 51(4): 615-622. |
| [10] | 贺大林,徐珊,郭鹏. 外泌体在泌尿系肿瘤精准诊断中的应用[J]. 北京大学学报(医学版), 2017, 49(4): 561-564. |
| [11] | 刘瑾,熊耕砚,唐琦,方冬,李学松,周利群. 上尿路尿路上皮癌中RASSF1A基因启动子区域的甲基化状态及其临床意义[J]. 北京大学学报(医学版), 2016, 48(4): 571-578. |
|
||