北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (6): 1113-1123. doi: 10.19723/j.issn.1671-167X.2025.06.015
肿瘤转移抑制基因LASS2去磷酸化对液泡型ATP酶活性及前列腺癌侵袭性的影响
刘艳华1, 陆敏1, 赵旭阳2, 张宽根1, 武睿1, 梅放1, 戴志豪1, 由江峰1, 裴斐1,*( )
)
- 1. 北京大学基础医学院病理学系/北京大学第三医院病理科, 北京 100191
2. 北京大学系统生物医学研究所, 北京 100191
Effect of dephosphorylation of tumor metastasis suppressor gene LASS2 on vacuolar ATPase activity and invasiveness of prostate cancer
Yanhua LIU1, Min LU1, Xuyang ZHAO2, Kuan'gen ZHANG1, Rui WU1, Fang MEI1, Zhihao DAI1, Jiangfeng YOU1, Fei PEI1,*( )
)
- 1. Department of Pathology, School of Basic Medical Sciences Peking University / Peking University Third Hospital, Beijing 100191, China
2. Peking University Institute of Systems Biomedicine, Beijing 100191, China
摘要:
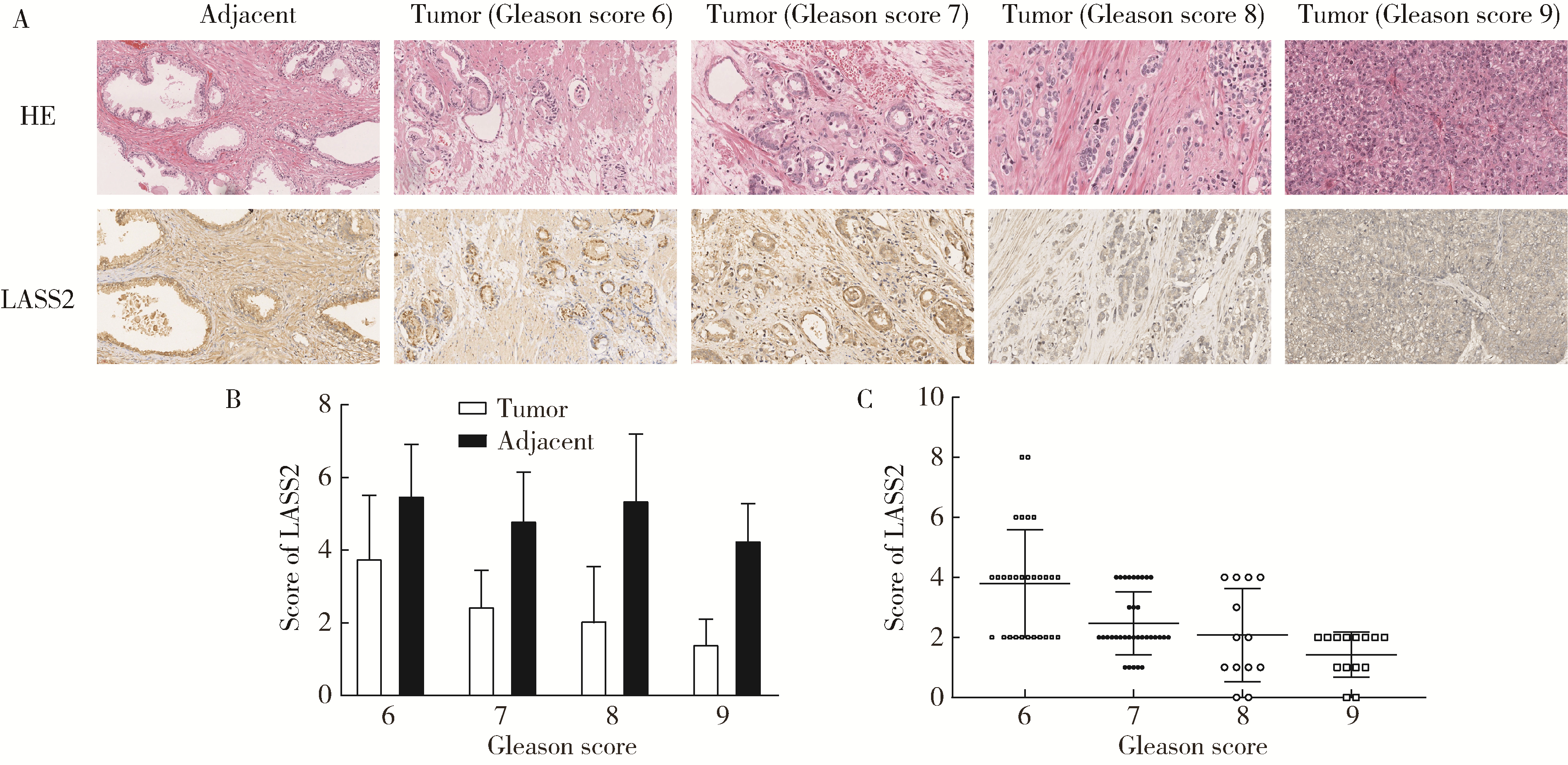
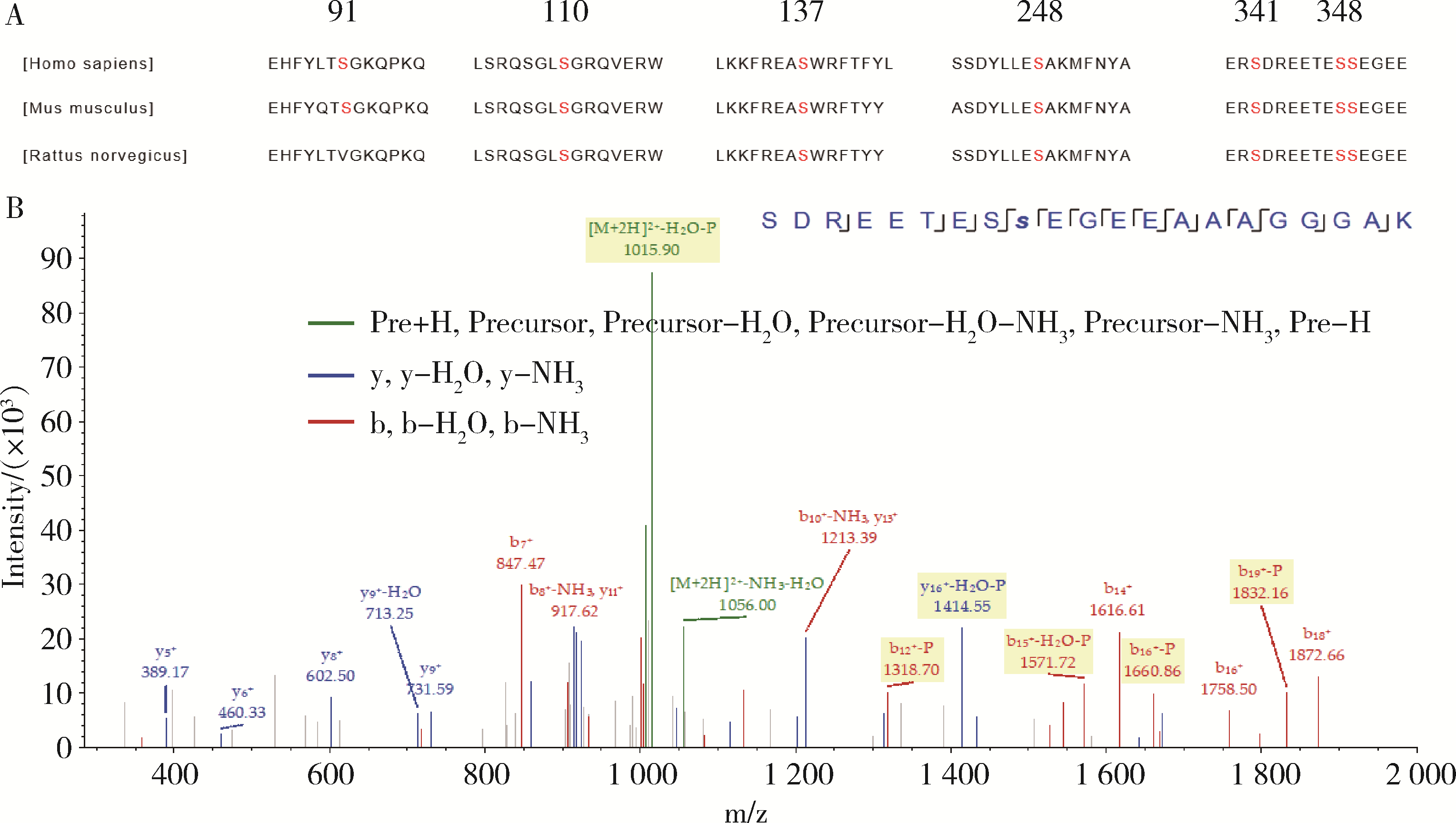
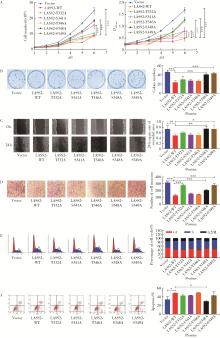
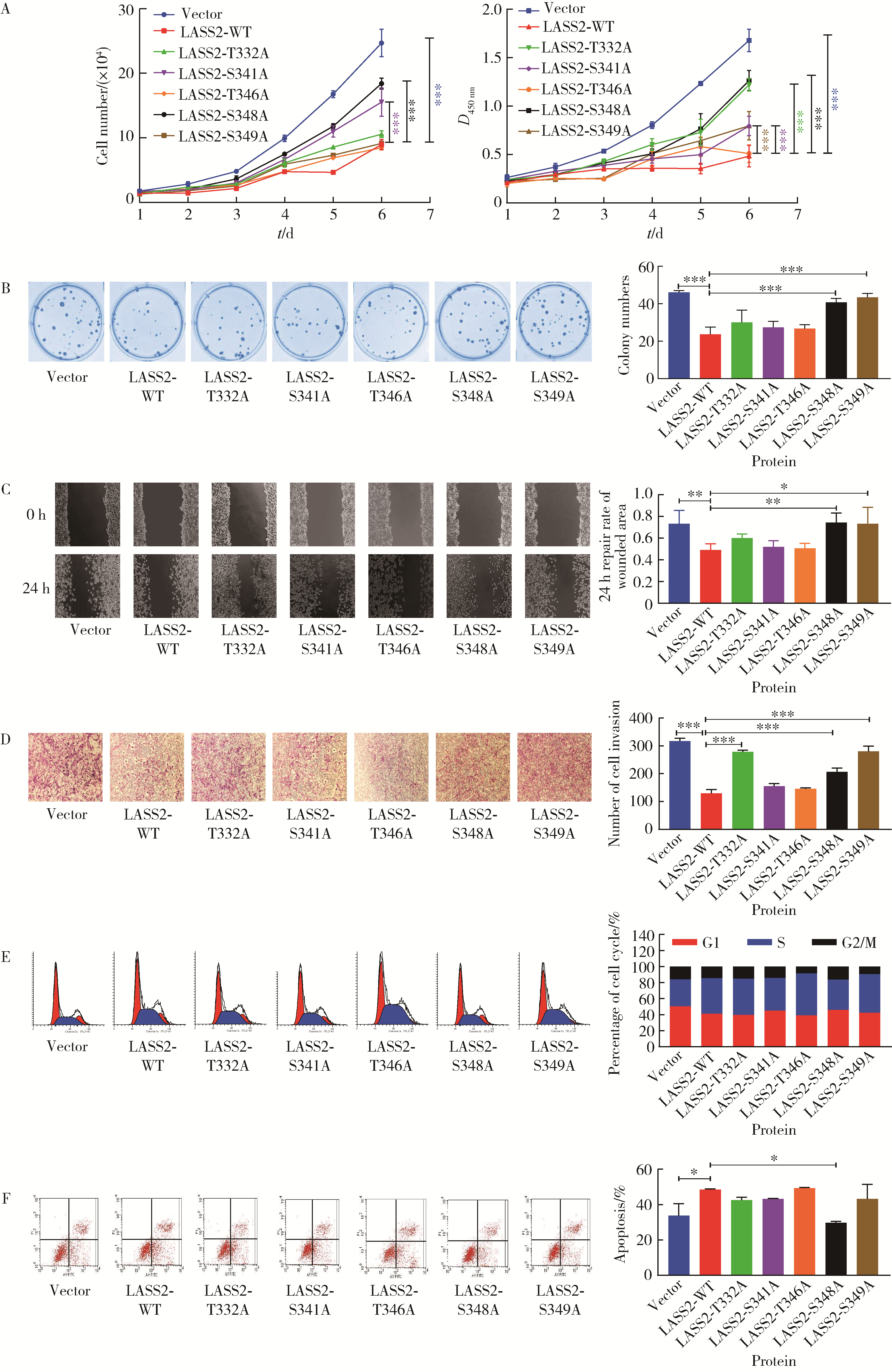
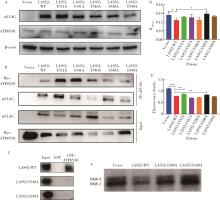
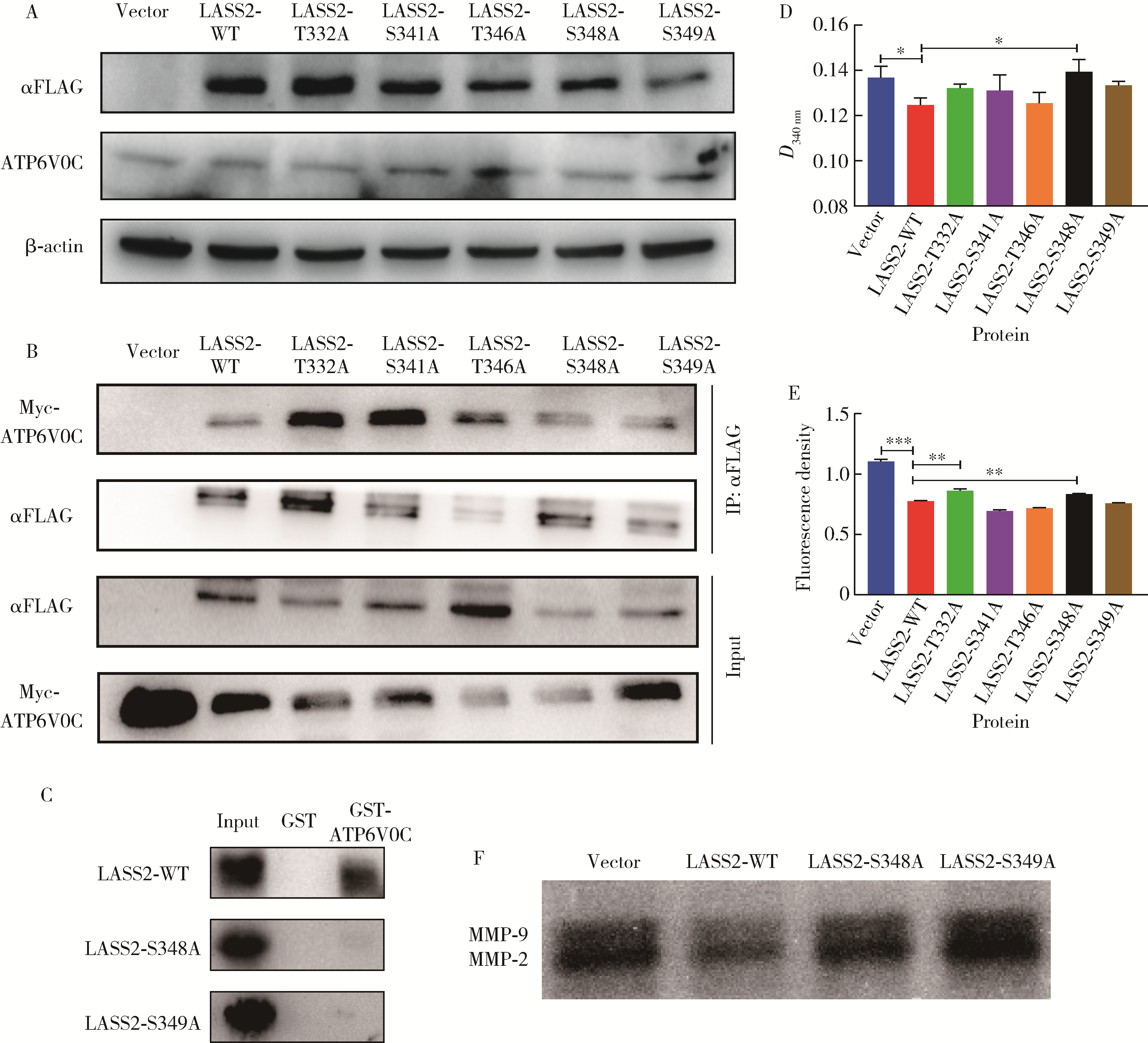
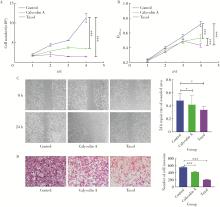
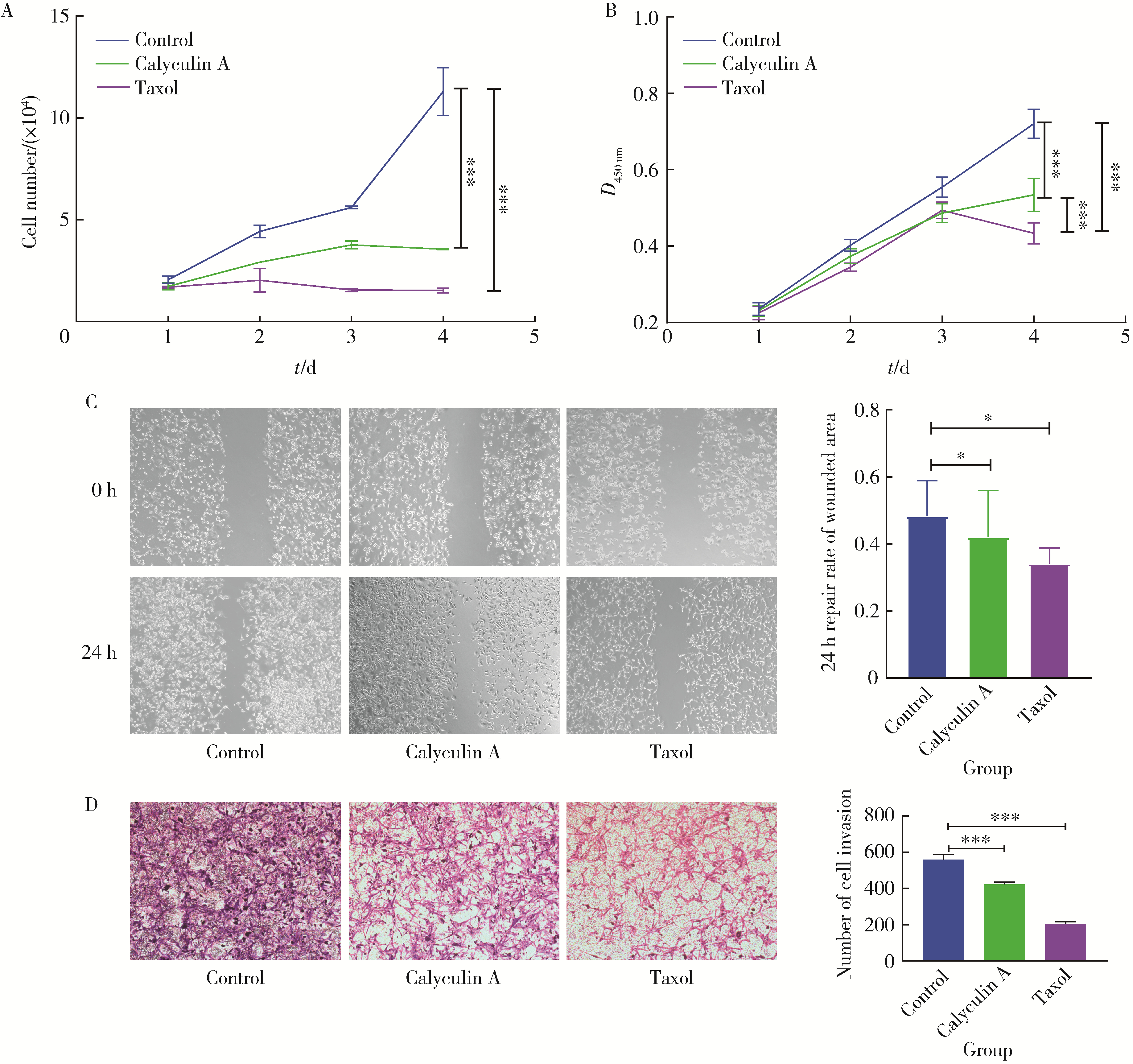
目的: 探讨人源酵母LAG1长寿同源物2(homo sapiens longevity assurance homolog 2 of yeast LAG1, LASS2)去磷酸化对前列腺癌细胞生物学功能的影响及其分子机制。方法: 对90例前列腺癌患者的石蜡组织芯片标本进行LASS2免疫组织化学染色, 并构建FLAG标签的LASS2全长pcDNA3真核表达载体, 转染至HEK 293T细胞后行免疫共沉淀和蛋白质谱检测, 分析LASS2的磷酸化位点。根据LASS2磷酸化位点结果构建LASS2 C末端5个去磷酸化突变体的pcDNA3真核表达载体, 并稳定转染到高转移潜能前列腺癌PC-3M-1E8细胞系中, 通过生长曲线、MTT掺入实验、平板克隆形成实验、细胞划痕实验、Transwell侵袭实验和流式细胞术检测LASS2及其去磷酸化突变体对前列腺癌细胞生物学功能的影响。检测LASS2及其突变体对液泡型ATP酶V0复合体中c亚基(ATP6V0C)表达量及两者相互结合的影响, 以及其对液泡型ATP酶活性、细胞外H+浓度和基质金属蛋白酶2(matrix metalloproteinase 2, MMP-2)活性的影响, 并探索蛋白磷酸酶抑制剂calyculin A对前列腺癌细胞增殖、迁移和侵袭能力的影响。结果: 免疫组织化学染色显示前列腺癌组织中LASS2的表达与Gleason分级呈负相关; 质谱分析发现LASS2的C末端存在3个磷酸化位点(Ser-341、Ser-348和Ser-349);与野生型LASS2相比, LASS2 S348位点的去磷酸化突变体(LASS2-S348A)能显著增强前列腺癌细胞的增殖能力、锚着不依赖生长能力、细胞迁移能力(细胞迁移率从49.11%±5.62%增加到74.28%±8.77%, P < 0.001)和侵袭能力(穿膜细胞数从129.67±13.65增加到206.67±13.50, P < 0.001), 并显著降低S期比例(从44.17%降低到37.90%, P < 0.05)和细胞凋亡率(从48.540%±0.269%降低到29.700%±0.778%, P < 0.05)。LASS2-S348A去磷酸化突变体虽然不影响ATP6V0C的蛋白表达量, 但会明显抑制LASS2与ATP6V0C的结合能力, 并进而显著增强液泡型ATP酶活性和细胞外H+浓度, 最终促进MMP-2的活性; 而蛋白磷酸酶抑制剂calyculin A能够显著抑制前列腺癌细胞的增殖、迁移和侵袭。结论: LASS2蛋白C末端S348位点的磷酸化对于LASS2的肿瘤抑制功能至关重要, 其分子机制可能是LASS2 S348位点的磷酸化通过增强其与ATP6V0C的结合而抑制液泡型ATP酶活性, 进而降低细胞外H+浓度和抑制MMP-2的活性, 并最终抑制前列腺癌细胞的侵袭; 蛋白磷酸酶抑制剂calyculin A有望成为侵袭性前列腺癌潜在的治疗药物。
中图分类号:
- R737.25
| 1 |
|
| 2 |
|
| 3 |
doi: 10.1360/02yc9061 |
| 4 |
|
| 5 |
doi: 10.1074/jbc.M707386200 |
| 6 |
doi: 10.1074/jbc.M111.280271 |
| 7 |
doi: 10.1002/jcb.29926 |
| 8 |
doi: 10.1074/jbc.M115.695858 |
| 9 |
doi: 10.1016/S0968-0004(02)02154-0 |
| 10 |
doi: 10.1074/jbc.M608092200 |
| 11 |
doi: 10.1126/science.aaz2924 |
| 12 |
doi: 10.1093/braincomms/fcab245 |
| 13 |
徐晓艳, 由江峰, 裴斐, 等. RNA沉默LASS2基因促进人前列腺癌细胞体外侵袭及其机制研究[J]. 北京大学学报(医学版), 2011, 43 (6): 814- 819.
doi: 10.3969/j.issn.1671-167X.2011.06.006 |
| 14 |
doi: 10.1002/jcb.24716 |
| 15 |
|
| 16 |
doi: 10.1074/jbc.M114.611210 |
| 17 |
doi: 10.1111/cas.14283 |
| 18 |
doi: 10.1002/jcb.24400 |
| 19 |
张宽根, 周雨禾, 邵雅昆, 等. 肿瘤转移抑制基因LASS2/TMSG1 S248A突变体通过增加ATP6V0C表达促进前列腺癌的侵袭[J]. 北京大学学报(医学版), 2019, 51 (2): 210- 220.
doi: 10.19723/j.issn.1671-167X.2019.02.003 |
| 20 |
|
| [1] | 杨小勇, 张帆, 马潞林, 刘承. 前列腺导管腺癌临床特征及腺外侵犯的影响因素[J]. 北京大学学报(医学版), 2025, 57(5): 956-960. |
| [2] | 宁家昕, 王浩然, 罗书航, 敬吉波, 王建业, 侯惠民, 刘明. 氧化应激相关基因与前列腺癌关系的多组学分析[J]. 北京大学学报(医学版), 2025, 57(4): 633-643. |
| [3] | 王泽远, 于栓宝, 郑浩轲, 陶金, 范雅峰, 张雪培. 基于临床特征和多参数MRI的前列腺癌盆腔淋巴结转移的术前预测模型[J]. 北京大学学报(医学版), 2025, 57(4): 684-691. |
| [4] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [5] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [6] | 姚凯烽,阮明健,李德润,田宇轩,陈宇珂,范宇,刘毅. 靶向穿刺联合区域系统穿刺对PI-RADS 4~5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2024, 56(4): 575-581. |
| [7] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [8] | 薛蔚,董樑,钱宏阳,费笑晨. 前列腺癌新辅助治疗与辅助治疗的现状及进展[J]. 北京大学学报(医学版), 2023, 55(5): 775-780. |
| [9] | 刘毅,袁昌巍,吴静云,沈棋,肖江喜,赵峥,王霄英,李学松,何志嵩,周利群. 靶向穿刺+6针系统穿刺对PI-RADS 5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2023, 55(5): 812-817. |
| [10] | 毛海,张帆,张展奕,颜野,郝一昌,黄毅,马潞林,褚红玲,张树栋. 基于MRI前列腺腺体相关参数构建腹腔镜前列腺癌术后尿失禁的预测模型[J]. 北京大学学报(医学版), 2023, 55(5): 818-824. |
| [11] | 袁昌巍,李德润,李志华,刘毅,山刚志,李学松,周利群. 多参数磁共振成像中动态对比增强状态在诊断PI-RADS 4分前列腺癌中的应用[J]. 北京大学学报(医学版), 2023, 55(5): 838-842. |
| [12] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [13] | 程晓静,蒋栋,张连海,王江华,李雅真,翟佳慧,闫宝琪,张露露,谢兴旺,李子禹,季加孚. KRAS G12V特异性T细胞受体治疗恶性肿瘤的临床前研究[J]. 北京大学学报(医学版), 2022, 54(5): 884-895. |
| [14] | 丁婷婷,曾楚雄,胡丽娜,余明华. 基于癌症基因组图谱数据库结直肠癌免疫细胞浸润预测模型的建立[J]. 北京大学学报(医学版), 2022, 54(2): 203-208. |
| [15] | 孙争辉,黄晓娟,董靖晗,刘茁,颜野,刘承,马潞林. 临床T1期肾细胞癌肾窦侵犯的危险因素[J]. 北京大学学报(医学版), 2021, 53(4): 659-664. |
|
||