北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (3): 541-547. doi: 10.19723/j.issn.1671-167X.2022.03.021
儿童坏死性肺炎临床特征及危险因素分析
钱婧1,魏友加1,程毅菁2,张奕1,彭博1,朱春梅1,*( )
)
- 1. 首都儿科研究所附属儿童医院呼吸内科,国家临床重点专科,北京 100020
2. 首都儿科研究所大数据中心,北京 100020
Analysis of clinical features and risk factors of necrotizing pneumonia in children
Jing QIAN1,You-jia WEI1,Yi-jing CHENG2,Yi ZHANG1,Bo PENG1,Chun-mei ZHU1,*( )
)
- 1. Department of Respiratory Medicine, Children's Hospital Affiliated to Capital Institute of Pediatrics, National Key Clinical Specialty, Beijing 100020, China
2. Big Data Center of Capital Institute of Pediatrics, Beijing 100020, China
摘要:
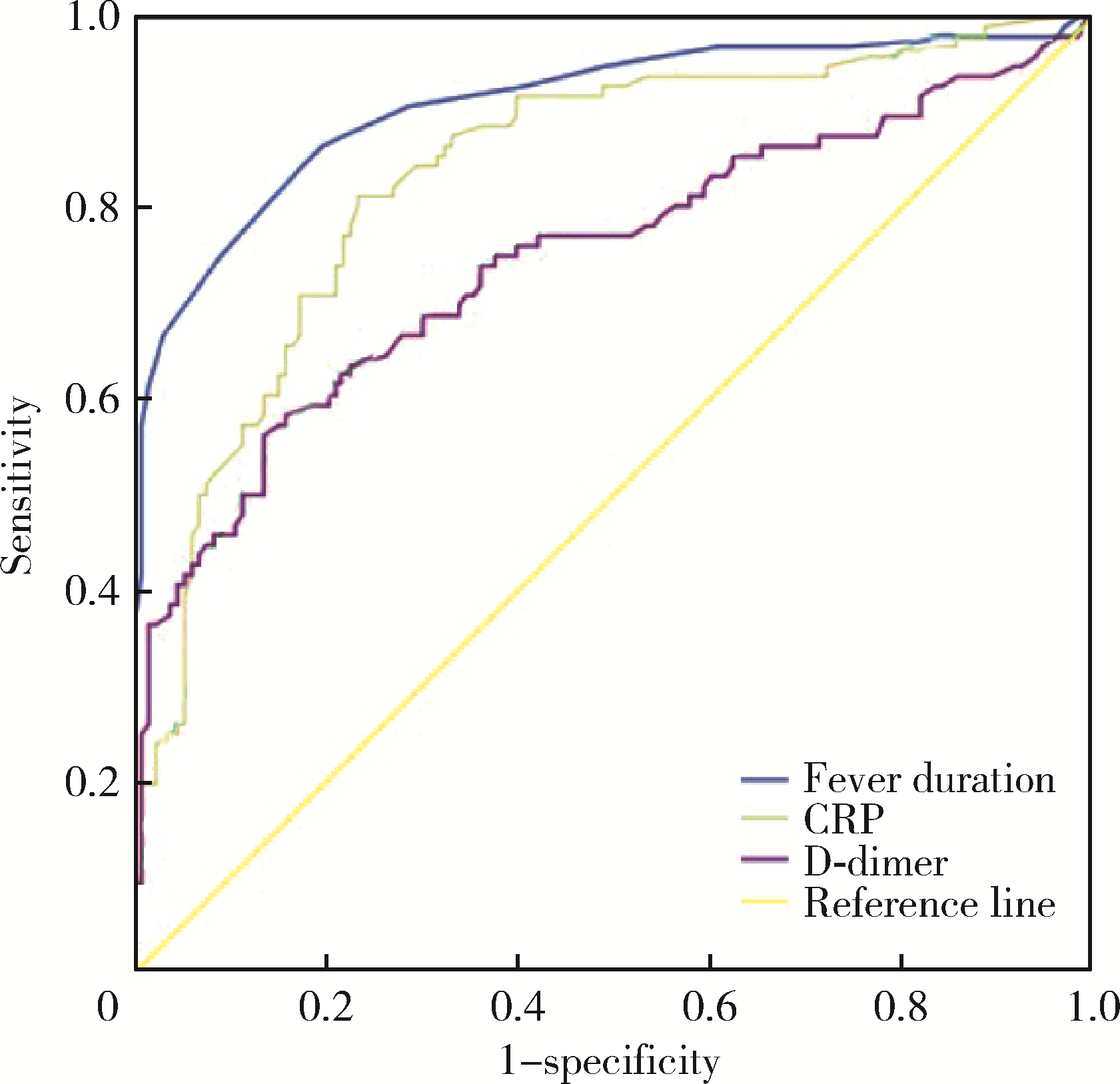
目的: 探讨儿童坏死性肺炎的临床特点及危险因素。方法: 回顾性分析2016年1月至2020年1月在首都儿科研究所附属儿童医院呼吸科住院的218例重症肺炎患儿的病例资料,根据是否发生肺坏死将患儿分为坏死性肺炎(necrotizing pneumonia, NP)组(96例)和非坏死性肺炎(non-necrotizing pneumonia, NNP)组(122例),比较两组临床特征(营养不良、热程、住院时间、影像学表现、治疗及转归随访情况)、实验室检查[白细胞、中性粒细胞比例、血小板计数(platelet,PLT)、C-反应蛋白(C-reactive protein,CRP)、降钙素原(procalcitonin,PCT)、D-二聚体(D-dimer)、乳酸脱氢酶(lactate dehydrogenase,LDH)]和支气管镜下表现的差异,对坏死性肺炎相关临床危险因素进行Logistic回归分析,进一步通过受试者工作特征(receiver operating characteristic, ROC)曲线确定各指标最大诊断价值的临界值。结果: 两组患儿在年龄、性别、病原学分类、支气管镜下表现的差异无统计学意义(P>0.05),NP组患儿的影像学吸收时间长于NNP组(P < 0.05)。两组患儿营养不良、热程、住院时间、白细胞计数、中性粒细胞比例、CRP、PCT、D-dimer等指标的差异有统计学意义(P < 0.05)。6岁以下患儿的影像学吸收时间短于6岁以上者,病程10 d内进行支气管肺泡灌洗治疗的患儿影像学吸收时间短于病程10 d以上者,混合感染组的影像学吸收时间明显长于单一病原感染组。对两组病例进行Logistic回归分析发现,热程、住院时间、CRP、PCT、D-dimer是继发肺坏死的危险因素(分别为P < 0.001、P < 0.001、P < 0.001、P=0.013、P=0.001)。绘制热程、CRP、PCT、D-dimer的ROC曲线,发现当热程>11.5 d、CRP>48.35 mg/L、D-dimer>4.25 mg/L时,对于预测肺坏死的发生有一定诊断价值[ROC曲线下面积(area under ROC curve, AUC)分别为0.909、0.836、0.747,P均 < 0.001]。结论: 儿童坏死性肺炎的热程、住院时间长,混合病原感染的影像学吸收时间明显长于单一病原感染;与6岁以上组患儿及病程>10 d组患儿相比,6岁以下以及病程10 d内的患儿行电子支气管镜肺泡灌洗的疗效更具优势;炎症指标CRP、PCT、D-dimer明显升高,热程、CRP、PCT、D-dimer是重症肺炎继发肺坏死的危险因素,热程>11.5 d、CRP>48.35 mg/L、D-dimer>4.25 mg/L对诊断坏死性肺炎有较高预测价值。
中图分类号:
- R725.6
| 1 |
Masters IB , Isles AF , Grimwood K . Necrotizing pneumonia: An emerging problem in children?[J]. Pneumonia (Nathan), 2017, 9, 11.
doi: 10.1186/s41479-017-0035-0 |
| 2 | Krenke K , Sanocki M , Urbankowska E , et al. Necrotizing pneumonia and its complications in children[J]. Adv Exp Med Biol, 2015, 857, 9- 17. |
| 3 |
Krutikov M , Rahman A , Tiberi S . Necrotizing pneumonia (aetiology, clinical features and management)[J]. Curr Opin Pulm Med, 2019, 25 (3): 225- 232.
doi: 10.1097/MCP.0000000000000571 |
| 4 | 戴菱蔓. 儿童坏死性肺炎的研究进展[J]. 国际儿科学杂志, 2021, 48 (3): 163- 167. |
| 5 |
Wang RS , Wang SY , Hsieh KS , et al. Necrotizing pneumonitis caused by Mycoplasma pneumoniae in pediatric patients: Report of five cases and review of literature[J]. Pediatr Infect Dis J, 2004, 23 (6): 564- 567.
doi: 10.1097/01.inf.0000130074.56368.4b |
| 6 | 宾松涛, 胡晓琴, 王继, 等. 儿童肺炎支原体坏死性肺炎30例临床分析[J]. 疑难病杂志, 2021, 20 (2): 144- 147. |
| 7 | 刘杰. 儿童坏死性肺炎临床特点分析[D]. 天津: 天津医科大学, 2020. |
| 8 |
杨男, 尚云晓. 儿童肺炎链球菌感染致坏死性肺炎的临床特点及预测指标研究[J]. 中华实用儿科临床杂志, 2020, 35 (8): 573- 577.
doi: 10.3760/cma.j.cn101070-20191015-00990 |
| 9 | 张园园, 戴菱蔓, 周云连, 等. 儿童细菌性坏死性肺炎与肺炎支原体坏死性肺炎临床特征及预后比较[J]. 中华儿科杂志, 2019, 57 (8): 625- 630. |
| 10 | 张天骄, 刘盈盈, 裴亮. 儿童肺炎支原体肺炎并发坏死性肺炎的临床预测因素[J]. 中国医科大学学报, 2022, 51 (1): 79- 82. |
| 11 |
杨男, 陈宁, 尚云晓. 儿童坏死性肺炎49例临床分析[J]. 中华实用儿科临床杂志, 2017, 32 (4): 280- 283.
doi: 10.3760/cma.j.issn.2095-428X.2017.04.010 |
| 12 | 刘帅帅, 马静, 张忠晓, 等. 增强CT对儿童坏死性肺炎的诊断价值[J]. 中华实用儿科临床杂志, 2021, 36 (4): 267- 270. |
| 13 | 曾洪武, 黄文献, 陈杰华, 等. 儿童坏死性肺炎的临床特点及胸部HRCT特征[J]. 放射学实践, 2018, 33 (7): 758- 761. |
| 14 | 杜雪平, 郭燕军. 儿童坏死性肺炎的临床特点及胸部CT特征[J]. 影像研究与医学应用, 2020, 4 (10): 68- 69. |
| 15 | Wang X , Zhong LJ , Chen ZM , et a1 . Necrotizing pneumonia caused by refractory Mycoplasma pneumonia pneumonia in children[J]. World J Pediatr, 2018, 14 (4): 344- 349. |
| 16 | 王敏敏. 儿童坏死性肺炎诊治进展[J]. 国际儿科学杂志, 2021, 48 (8): 529- 533. |
| 17 | 席少婷, 蔡栩栩. 儿童难治性肺炎支原体肺炎诊治进展[J]. 国际儿科学杂志, 2020, 47 (6): 384- 388. |
| 18 | Takigawa Y , Fujiwara K , Saito T , et al. Rapidly progressive multiple cavity formation in necrotizing pneumonia caused by community-acquired methicillin-resistant Staphylococcus aureus positive for the Panton-Valentine leucocidin gene[J]. Intern Med, 2019, 58 (5): 685- 691. |
| 19 | 王晓丽, 郑兴厂, 管栋, 等. 胸部CT及可弯曲支气管镜在坏死性肺炎的应用价值[J]. 中国小儿急救医学, 2020, 27 (11): 830- 833. |
| 20 | 陈鲁闽, 王程毅, 宋朝敏, 等. 重症肺炎患儿凝血指标与危重症评分的相关性分析[J]. 中国小儿急救医学, 2013, 20 (4): 380- 382. |
| 21 | Graw-Panzer KD , Verma S , Rao S , et al. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae[J]. J Natl Med Assoc, 2009, 101 (9): 956- 958. |
| 22 | 刘金荣, 徐保平, 李惠民, 等. 肺炎链球菌坏死性肺炎20例诊治分析[J]. 中华儿科杂志, 2012, 50 (6): 431- 434. |
| 23 | 贺艺璇, 张春峰, 吴润晖, 等. D-二聚体在肺炎支原体肺炎患儿病情及预后判断中的应用[J]. 中华实用儿科临床杂志, 2019, 34 (22): 1702- 1706. |
| 24 | 刘帅帅, 马静, 张忠晓, 等. 儿童肺炎支原体坏死性肺炎的早期预测指标[J]. 中华实用儿科临床杂志, 2021, 36 (8): 601- 604. |
| [1] | 赵双云, 邹思雨, 李雪莹, 沈丽娟, 周虹. 中文版口腔健康素养量表简版(HeLD-14)在学龄前儿童家长中应用的信度和效度评价[J]. 北京大学学报(医学版), 2024, 56(5): 828-832. |
| [2] | 陈心心, 唐哲, 乔艳春, 荣文笙. 北京市密云区4岁儿童患龋状况及其与龋活跃性检测的相关性[J]. 北京大学学报(医学版), 2024, 56(5): 833-838. |
| [3] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [4] | 颜野,李小龙,夏海缀,朱学华,张羽婷,张帆,刘可,刘承,马潞林. 前列腺癌根治术后远期膀胱过度活动症的危险因素[J]. 北京大学学报(医学版), 2024, 56(4): 589-593. |
| [5] | 陈延,李况蒙,洪锴,张树栋,程建星,郑仲杰,唐文豪,赵连明,张海涛,姜辉,林浩成. 阴茎海绵体注射试验对阴茎血管功能影响的回顾性研究[J]. 北京大学学报(医学版), 2024, 56(4): 680-686. |
| [6] | 庞博,郭桐君,陈曦,郭华棋,石嘉章,陈娟,王欣梅,李耀妍,单安琪,余恒意,黄婧,汤乃军,王艳,郭新彪,李国星,吴少伟. 天津与上海35岁以上人群氮氧化物个体暴露水平及其影响因素[J]. 北京大学学报(医学版), 2024, 56(4): 700-707. |
| [7] | 和静,房中则,杨颖,刘静,马文瑶,霍勇,高炜,武阳丰,谢高强. 血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系[J]. 北京大学学报(医学版), 2024, 56(4): 722-728. |
| [8] | 岳芷涵,韩娜,鲍筝,吕瑾莨,周天一,计岳龙,王辉,刘珏,王海俊. 儿童早期体重指数轨迹与超重风险关联的前瞻性队列研究[J]. 北京大学学报(医学版), 2024, 56(3): 390-396. |
| [9] | 蔡珊,张依航,陈子玥,刘云飞,党佳佳,师嫡,李佳欣,黄天彧,马军,宋逸. 北京市中小学生身体活动时间现状及影响因素的路径[J]. 北京大学学报(医学版), 2024, 56(3): 403-410. |
| [10] | 张祖洪,陈天娇,马军. 中小学生青春发动时相与心血管代谢危险因素的相关性[J]. 北京大学学报(医学版), 2024, 56(3): 418-423. |
| [11] | 林郁婷,王华丽,田宇,巩俐彤,常春. 北京市老年人认知功能的影响因素[J]. 北京大学学报(医学版), 2024, 56(3): 456-461. |
| [12] | 费秀文,刘斯,汪波,董爱梅. 成人及儿童组织坏死性淋巴结炎临床特征及治疗[J]. 北京大学学报(医学版), 2024, 56(3): 533-540. |
| [13] | 朱金荣,赵亚娜,黄巍,赵微微,王悦,王松,苏春燕. 感染新型冠状病毒的血液透析患者的临床特征[J]. 北京大学学报(医学版), 2024, 56(2): 267-272. |
| [14] | 赖展鸿,李嘉辰,贠泽霖,张永刚,张昊,邢晓燕,邵苗,金月波,王乃迪,李依敏,李玉慧,栗占国. 特发性炎性肌病完全临床应答相关因素的单中心真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 284-292. |
| [15] | 司筱芊,赵秀娟,朱凤雪,王天兵. 创伤出血性休克后急性呼吸窘迫综合征的危险因素[J]. 北京大学学报(医学版), 2024, 56(2): 307-312. |
|
||