北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (4): 735-740. doi: 10.19723/j.issn.1671-167X.2022.04.024
环磷酰胺诱导SD大鼠膀胱疼痛综合征模型的有效性
- 北京大学人民医院泌尿外科,北京 100044
Urodynamic and histological evaluation of cyclophosphamide-induced bladder pain syndrome in SD rats
Lin ZHU,Wei-yu ZHANG,Ke-xin XU*( )
)
- Department of Urology, Peking University People's Hospital, Beijing 100044, China
摘要:
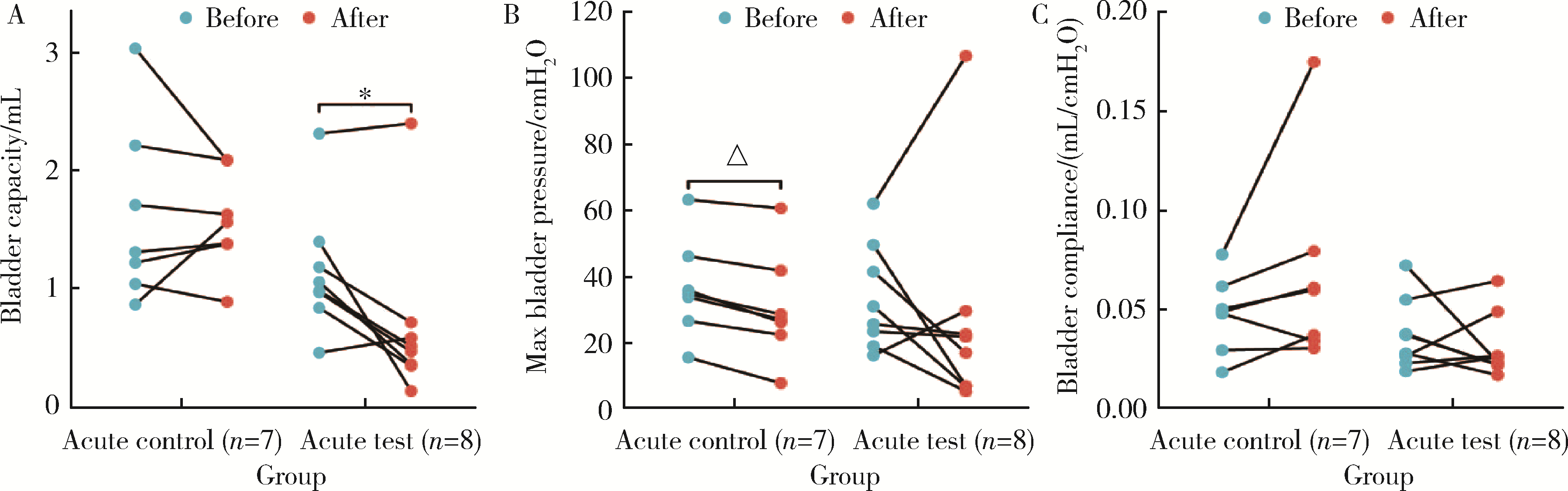
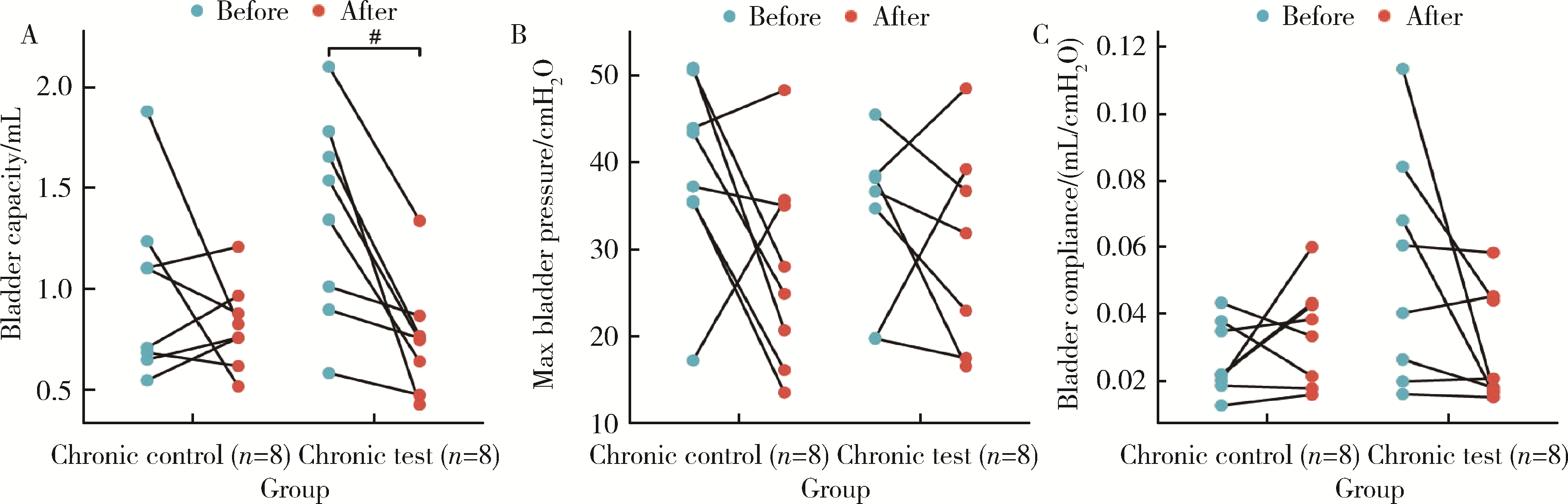
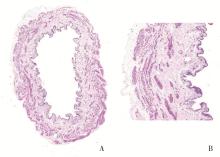
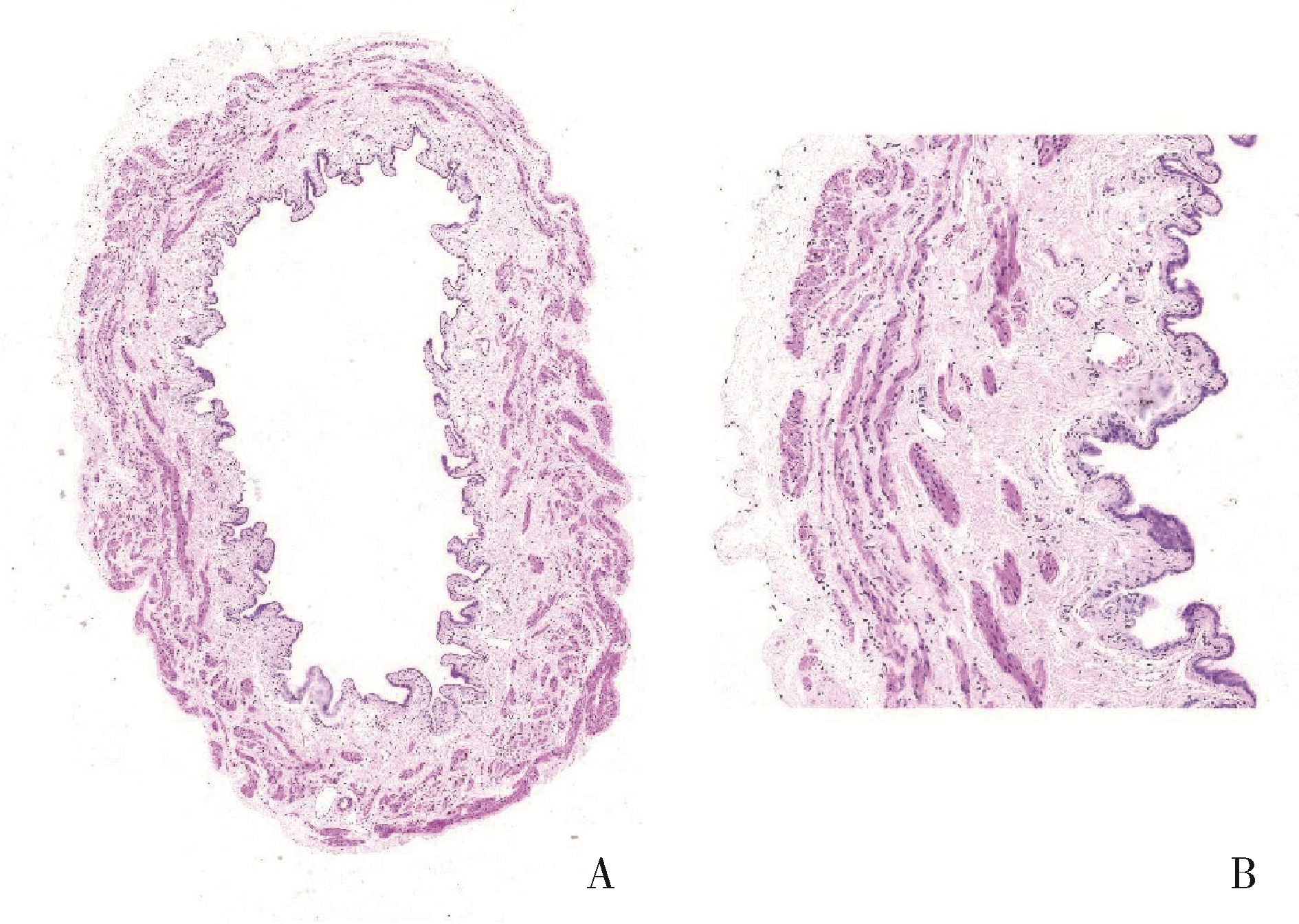
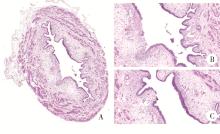
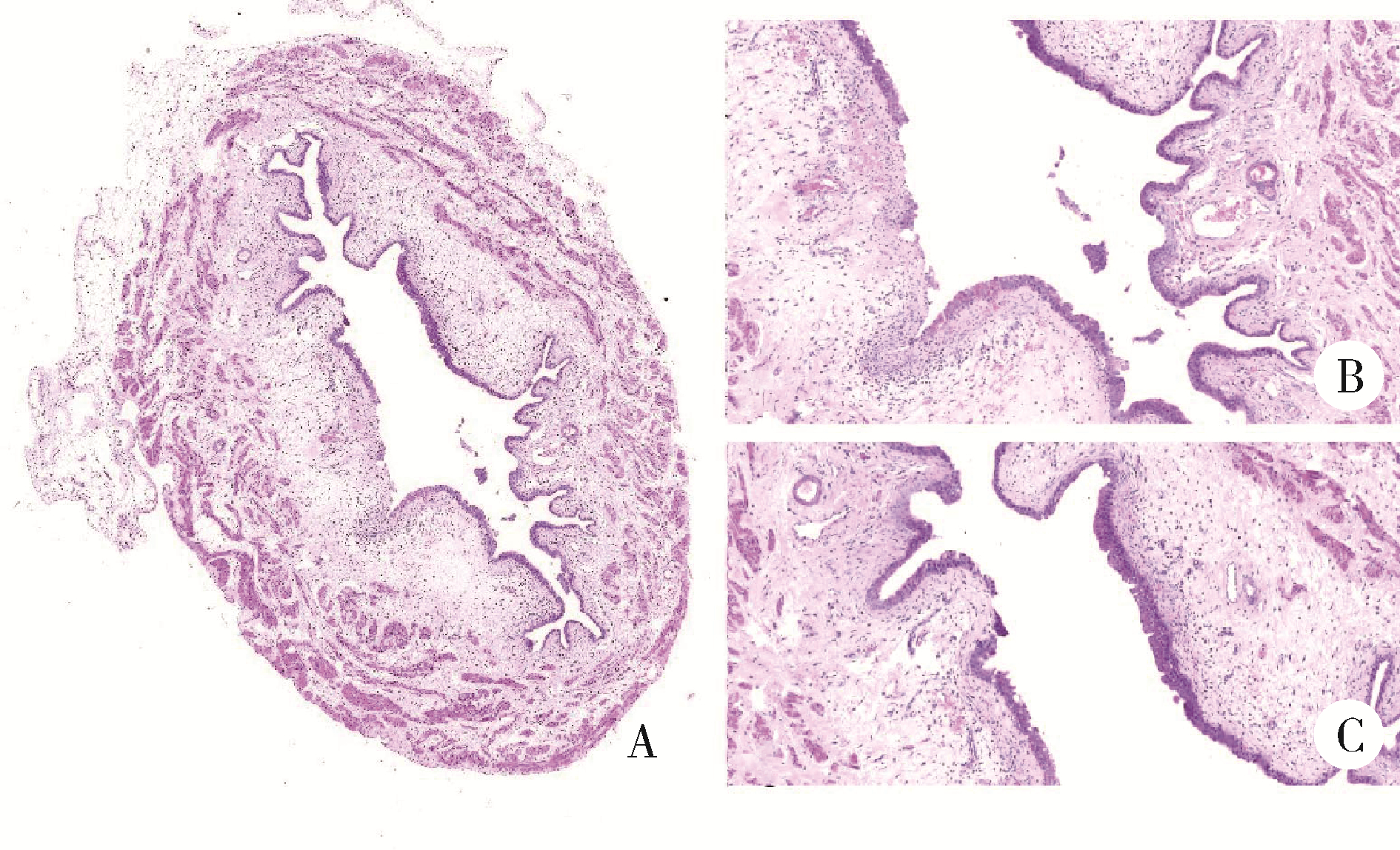
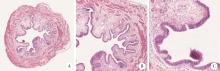
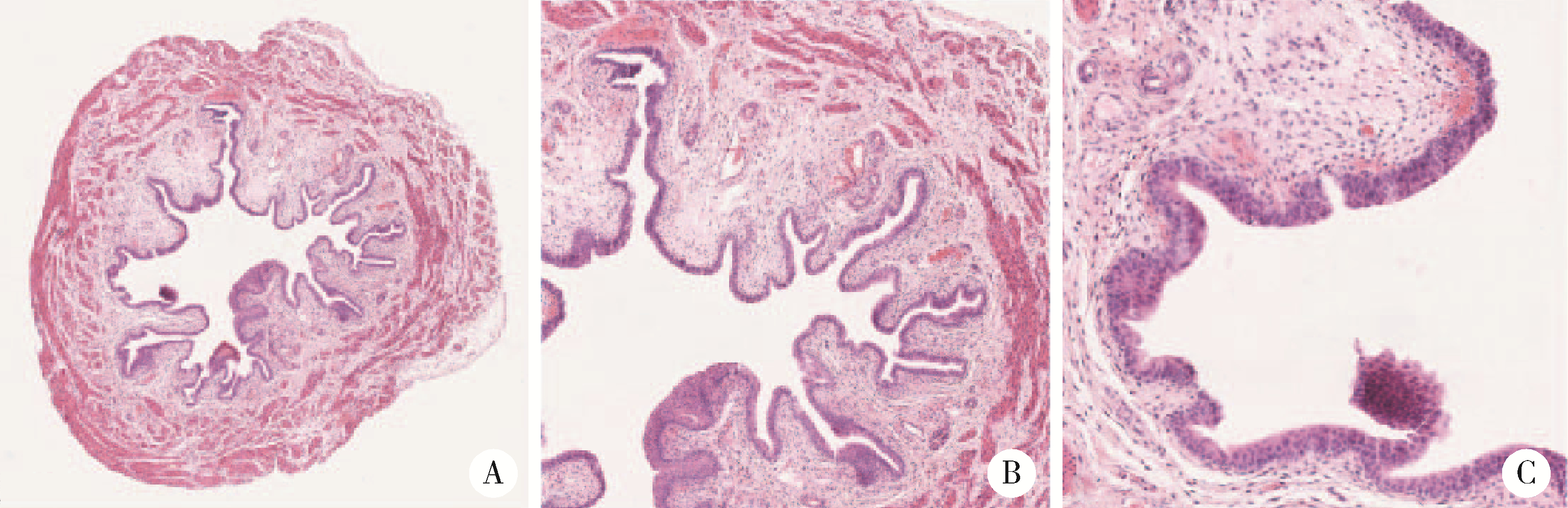
目的: 用环磷酰胺腹腔注射的方法建立SD大鼠膀胱疼痛综合征模型,从尿动力学与组织学层面评估模型的有效性,为膀胱疼痛综合征的临床研究奠定动物学基础,并进一步指导临床治疗。方法: 将32只8周龄SD大鼠随机分为4组,包括急性实验组、急性对照组、慢性实验组、慢性对照组,每组8只。急性实验组在第1天测完尿动力学数据后即刻行腹腔注射环磷酰胺150 mg/kg,第3天再次行尿动力学检查,之后处死大鼠,获取膀胱组织。慢性实验组第1天测量尿动力基线数据后,在第1、4、7天腹腔注射环磷酰胺75 mg/kg,第8天再次测量尿动力学数据后处死大鼠,得到膀胱组织。急性对照组与慢性对照组在腹腔注射等量生理盐水,尿动力学测量时间点与对应的实验组一致。苏木精-伊红(hematoxylin-eosin staining,HE)染色评估膀胱组织病理学改变。结果: 急性与慢性每组的对照组和实验组的尿动力学基线水平差异无统计学意义。急性实验组给药后尿动力学最大膀胱容量显著减小(t=-2.961, P < 0.05),组织学可看到严重的间质水肿、明显的炎性细胞浸润、黏膜水肿和黏膜下出血,部分尿路上皮缺失,符合急性膀胱炎表现。慢性实验组给药后可看到尿动力学最大膀胱容量显著减小(t=-3.886, P < 0.05),膀胱顺应性较对照组降低,但差异无统计学意义,慢性实验组组织学表现为尿路上皮剥脱、间质水肿、黏膜下出血和淋巴细胞等炎性细胞浸润,血管分布密集。结论: 急性实验组单次腹腔注射环磷酰胺可诱导大鼠产生膀胱疼痛综合征急性发作的膀胱炎症表现,慢性实验组反复注射环磷酰胺可诱导大鼠产生慢性膀胱疼痛综合征慢性炎症的组织学改变,但急性与慢性实验组膀胱功能并未出现明显受损。
中图分类号:
- R694.5
| 1 | Hakimi Z , Houbiers J , Pedersini R , et al. The burden of bladder pain in five European countries: a cross-sectional study[J]. Urology, 2017, 99 (1): 84- 91. |
| 2 |
Hanno P , Lin A , Nordling J , et al. Bladder pain syndrome committee of the International Consultation on Incontinence[J]. Neurourol Urodyn, 2010, 29 (1): 191- 198.
doi: 10.1002/nau.20847 |
| 3 |
Homma Y , Akiyama Y , Tomoe H , et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome[J]. Int J Urol, 2020, 27 (7): 578- 589.
doi: 10.1111/iju.14234 |
| 4 | Akiyama Y , Hanno P . Phenotyping of interstitial cystitis/bladder pain syndrome[J]. Int J Urol, 2019, 26 (Suppl 1): 17- 19. |
| 5 | Chai TC , Russo A , Yu S , et al. Mucosal signaling in the bladder[J]. Auton Neurosci, 2016, 200 (10): 49- 56. |
| 6 |
Karamali M , Shafabakhsh R , Ghanbari Z , et al. Molecular pathogenesis of interstitial cystitis/bladder pain syndrome based on gene expression[J]. J Cell Physiol, 2019, 234 (8): 12301- 12308.
doi: 10.1002/jcp.28009 |
| 7 | Lee MH , Wu HC , Tseng CM , et al. Health education and symptom flare management using a video-based health system for caring women with BPS/IC[J]. Urology, 2018, 119 (9): 62- 69. |
| 8 | Crescenze IM , Tucky B , Li J , Moore C , et al. Efficacy, side effects, and monitoring of oral cyclosporine in interstitial cystitis-bladder pain syndrome[J]. Urology, 2017, 107 (9): 49- 54. |
| 9 |
杨进益, 魏伟, 叶林, 等. 膀胱水扩张后透明质酸钠灌注治疗间质性膀胱炎疗效分析[J]. 中华泌尿外科杂志, 2012, 33 (3): 219- 222.
doi: 10.3760/cma.j.issn.1000-6702.2012.03.019 |
| 10 |
Ryu CM , Yu HY , Lee HY , et al. Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome[J]. Theranostics, 2018, 8 (20): 5610- 5624.
doi: 10.7150/thno.27559 |
| 11 |
Lv YS , Yao YS , Rong L , et al. Intravesical hyaluronidase causes chronic cystitis in a rat model: a potential model of bladder pain syndrome/interstitial cystitis[J]. Int J Urol, 2014, 21 (6): 601- 607.
doi: 10.1111/iju.12358 |
| 12 |
Mills KA , Chess-Williams R , McDermott C . Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde's contribution to urothelial dysfunction in vitro[J]. Arch Toxicol, 2019, 93 (11): 3291- 3303.
doi: 10.1007/s00204-019-02589-1 |
| 13 |
de Oliveira MG , Mónica FZ , Calmasini FB , et al. Deletion or pharmacological blockade of TLR4 confers protection against cyclophosphamide-induced mouse cystitis[J]. Am J Physiol Renal Physiol, 2018, 315 (3): 460- 468.
doi: 10.1152/ajprenal.00100.2018 |
| 14 | Augé C , Gamé X , Vergnolle N , et al. Characterization and validation of a chronic model of cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in rats[J]. Front Pharmacol, 2020, 11 (8): 1305. |
| 15 |
Yang W , Yaggie RE , Jiang MC , et al. Acyloxyacyl hydrolase modulates pelvic pain severity[J]. Am J Physiol Regul Integr Comp Physiol, 2018, 314 (3): 353- 365.
doi: 10.1152/ajpregu.00239.2017 |
| 16 | Lee UJ , Ackerman AL , Wu A , Zhang R , et al. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia[J]. Physiol Behav, 2015, 139 (2): 541- 548. |
| 17 |
Akiyama Y , Luo Y , Hanno PM , et al. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives[J]. Int J Urol, 2020, 27 (6): 491- 503.
doi: 10.1111/iju.14229 |
| 18 |
Birder L , Andersson KE . Animal modelling of interstitial cystitis/bladder pain syndrome[J]. Int Neurourol J, 2018, 22 (Suppl 1): 3- 9.
doi: 10.5213/inj.1835062.531 |
| [1] | 柯涵炜, 王起, 许克新. 优化环磷酰胺剂量在间质性膀胱炎/膀胱疼痛综合征啮齿动物模型中的应用[J]. 北京大学学报(医学版), 2024, 56(5): 908-912. |
| [2] | 辛鹏,张昊,姜振明. 膀胱内灌注电灼联合水扩张法治疗女性间质性膀胱炎[J]. 北京大学学报(医学版), 2023, 55(5): 865-870. |
| [3] | 孟令玮,李雪,高胜寒,李悦,曹瑞涛,张毅,潘韶霞. 三种方法建立大鼠种植体周炎模型的比较[J]. 北京大学学报(医学版), 2023, 55(1): 22-29. |
| [4] | 邵苗,郭惠芳,雷玲彦,赵清,丁艳杰,林进,吴锐,于峰,李玉翠,苗华丽,张莉芸,杜燕,焦瑞英,庞丽霞,龙丽,栗占国,李茹. 短间期小剂量环磷酰胺治疗系统性红斑狼疮耐受性的多中心对照研究[J]. 北京大学学报(医学版), 2022, 54(6): 1112-1116. |
| [5] | 王贵红,左婷,李然,左正才. 瑞巴派特在大鼠痛风性关节炎急性发作中的作用[J]. 北京大学学报(医学版), 2021, 53(4): 716-720. |
| [6] | 王佳文,刘敬超,孟令峰,张威,刘晓东,张耀光. 间质性膀胱炎/膀胱疼痛综合征患者生活质量及相关因素分析[J]. 北京大学学报(医学版), 2021, 53(4): 653-658. |
| [7] | 王涛,许克新,张维宇,胡浩,张晓威,王焕瑞,刘献辉,陈京文,张晓鹏. 男性膀胱过度活动症的尿动力学分型及临床疗效随访[J]. 北京大学学报(医学版), 2019, 51(6): 1048-1051. |
| [8] | 张维宇,夏秋翔,胡浩,陈京文,孙屹然,许克新,张晓鹏. 门诊女性下尿路症状患者尿动力学检查结果分析及逼尿肌无力患者的随访[J]. 北京大学学报(医学版), 2019, 51(5): 856-862. |
| [9] | 张维宇,张晓鹏,陈京文,孙屹然,王佳,胡浩,许克新. 年龄因素对女性尿失禁患者尿动力学参数的影响[J]. 北京大学学报(医学版), 2016, 48(5): 825-829. |
| [10] | 张维宇,胡浩,王起,陈京文,许克新. 女性压力性尿失禁患者术前尿动力学检查的意义[J]. 北京大学学报(医学版), 2016, 48(4): 655-658. |
| [11] | 许丹, 林峰, 朱小语, 刘文颖, 陈晓文, 冯金秋, 范爱琴, 蔡木易, 许雅君. 牡蛎肽对免疫抑制小鼠免疫功能的影响[J]. 北京大学学报(医学版), 2016, 48(3): 392-397. |
| [12] | 刘宁, 何峰, 满立波, 黄广林, 王海东, 王海, 李贵忠, 王建伟. 尿动力学检查中伪像的曲线特征及影响因素分析[J]. 北京大学学报(医学版), 2014, 46(5): 817-820. |
| [13] | 邓小林, 张虎, 史本涛, 关志忱. 家庭尿流率测定在下尿路症状患者评估中的研究进展[J]. 北京大学学报(医学版), 2012, 44(4): 655-658. |
| [14] | 关志忱, 邓小林, 张黔. 新型移动式家庭电子尿流率仪和Laborie 尿流率仪临床检测结果比较[J]. 北京大学学报(医学版), 2011, 43(4): 616-619. |
| [15] | 胡卫国, 王晓峰, 徐涛, 李建兴, 陈亮, 于澄钒, 黄晓波. 纳米细菌大鼠肾结石模型初步建立及成石因素分析[J]. 北京大学学报(医学版), 2010, 42(4): 433-435. |
|
||