北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (4): 650-661. doi: 10.19723/j.issn.1671-167X.2025.04.004
TFE3重排肾细胞癌合并静脉癌栓患者的临床病理特征及生存分析
张展奕1,*, 陆敏2,3,*, 孙悦皓1, 董靖晗1, 侯小飞1, 肖春雷1, 王国良1, 田晓军1, 马潞林1, 张洪宪1, 张树栋1,4,*( )
)
- 1. 北京大学第三医院泌尿外科, 北京 100191
2. 北京大学第三医院病理科, 北京 100191
3. 北京大学基础医学院病理学系, 北京 100191
4. 北京大学第三医院肿瘤中心, 北京 100191
Clinicopathological features and survival analysis of TFE3-rearranged renal cell carcinoma with venous tumor thrombus
Zhanyi ZHANG1, Min LU2,3, Yuehao SUN1, Jinghan DONG1, Xiaofei HOU1, Chunlei XIAO1, Guoliang WANG1, Xiaojun TIAN1, Lulin MA1, Hongxian ZHANG1, Shudong ZHANG1,4,*( )
)
- 1. Department of Urology, Peking University Third Hospital, Beijing 100191, China
2. Department of Pathology, Peking University Third Hospital, Beijing 100191, China
3. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191, China
4. Cancer Center, Peking University Third Hospital, Beijing 100191, China
摘要:
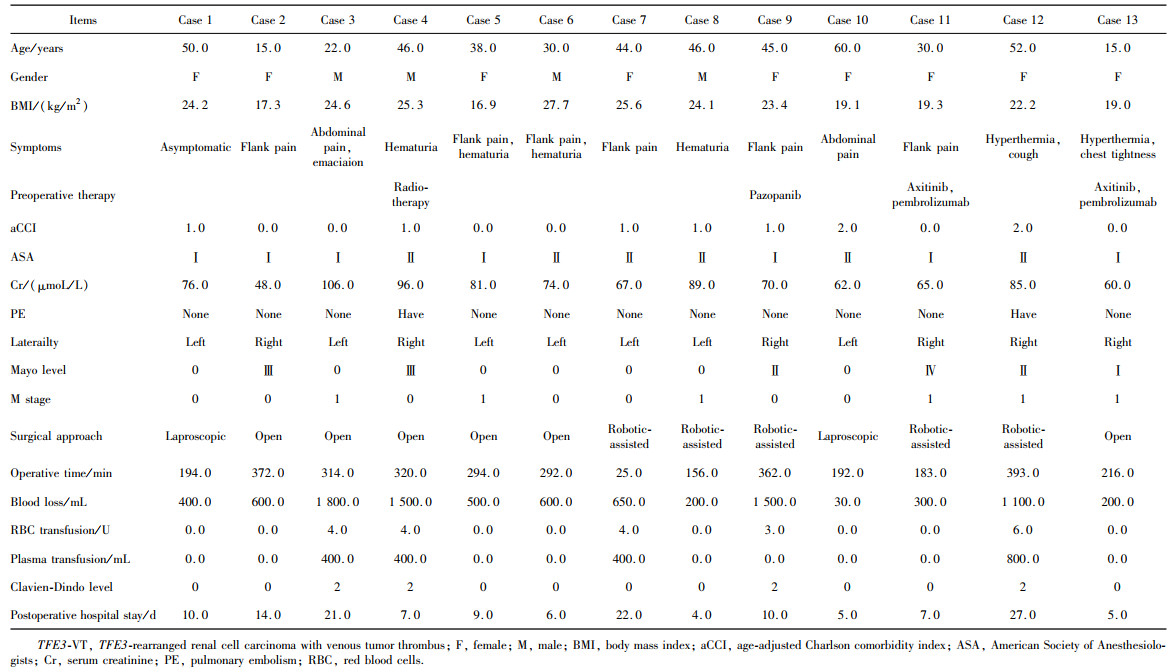
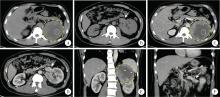
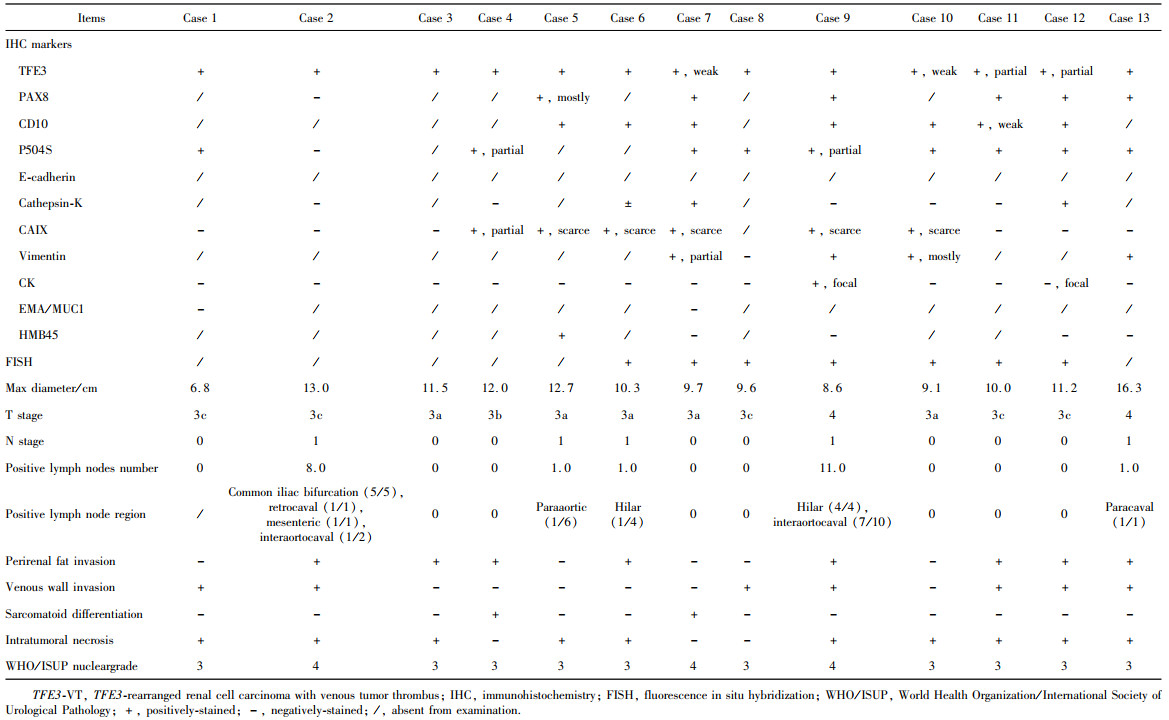
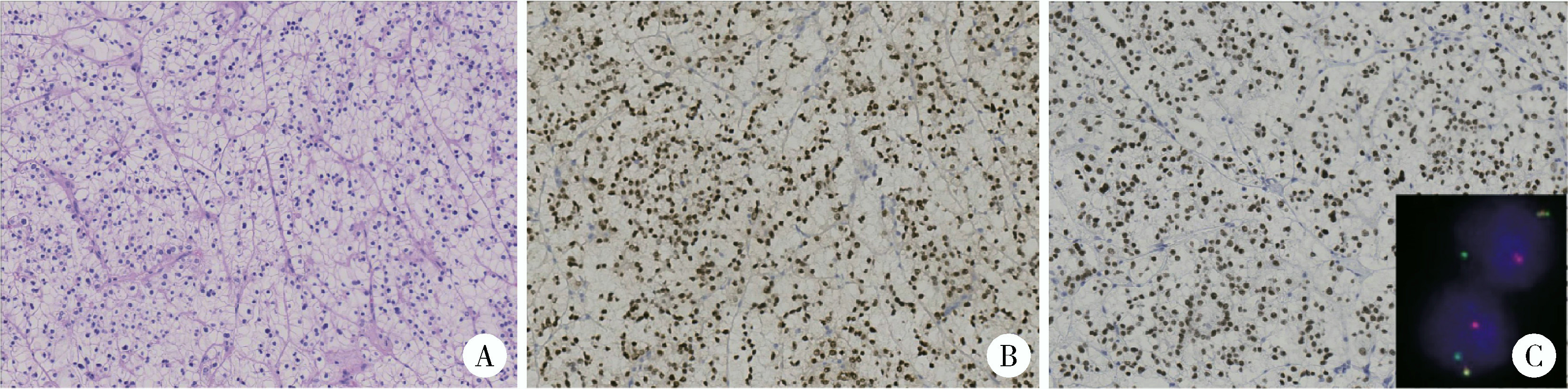
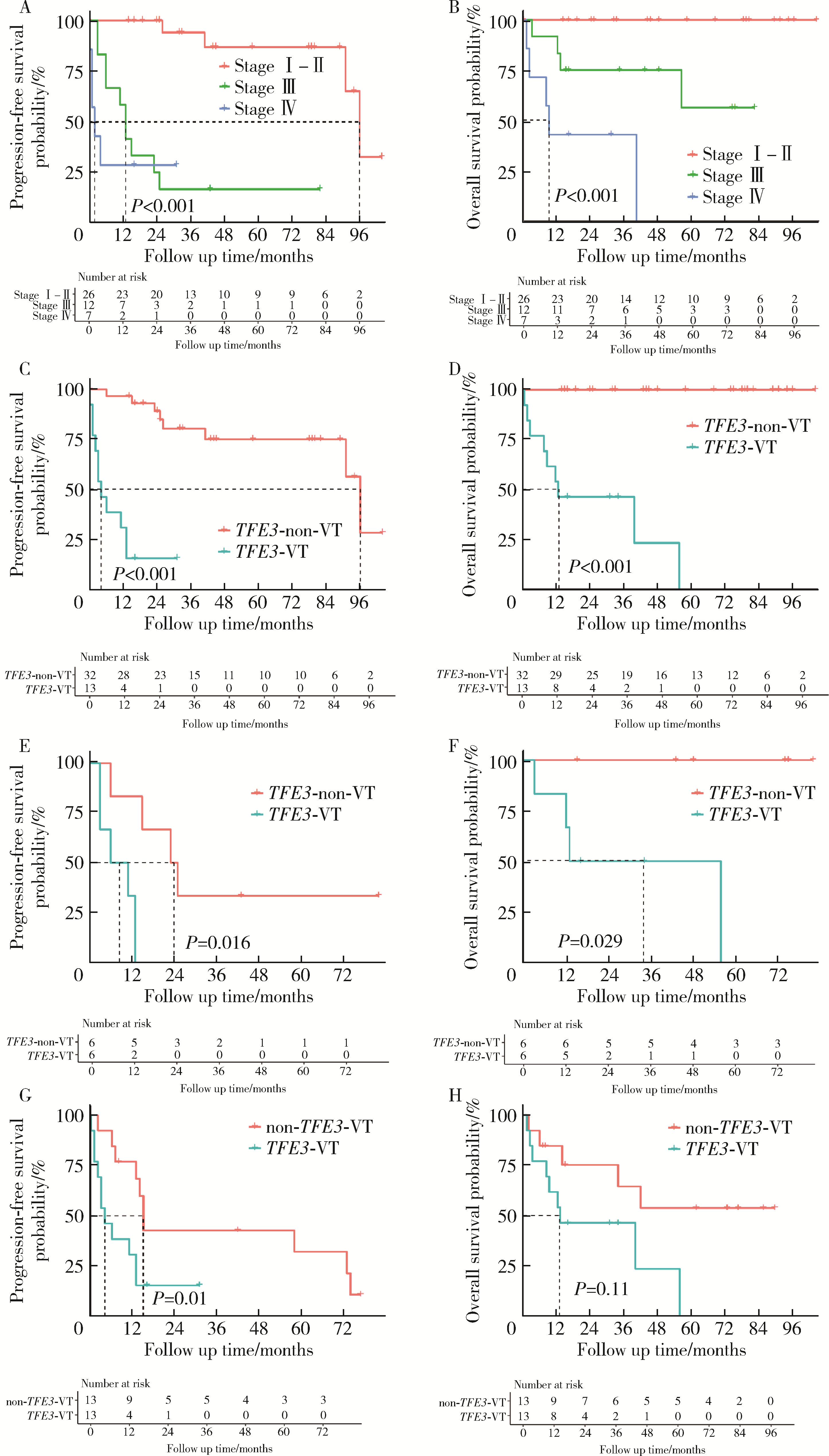
目的: 回顾TFE3重排肾细胞癌(TFE3-rearranged renal cell carcinoma, TFE3-RCC)合并静脉癌栓(venous tumor thrombus, VT)(TFE3-VT)患者的临床病理特征, 探索其治疗策略及预后特点, 为TFE3-VT患者的诊断和治疗提供参考。方法: 纳入2013年1月至2024年1月在北京大学第三医院泌尿外科接受手术且术后病理诊断为TFE3-VT的患者、诊断为TFE3-RCC但不合并VT(TFE3-non-VT)的患者, 以及诊断为非TFE3突变的肾细胞癌且合并VT(non-TFE3-VT)的患者。收集患者的临床病史资料、影像学资料、病理资料及随访资料。研究的主要结局为无进展生存期(progression free survival, PFS), 次要结局为总生存期(overall survival, OS)。(1)对TFE3-VT与TFE3-non-VT患者的基线资料进行比较, 服从正态分布的连续变量以均数±标准差表示, 组间比较采用Student’s t检验; 不服从正态分布的连续变量以中位数(P25, P75)表示, 组间比较采用Mann-Whitney U检验; 分类变量以样本数及百分比[n(%)]表示, 组间比较采用χ2检验或Fisher’s精确检验。(2)对13例TFE3-VT患者的临床病史、影像学表现、手术情况及组织病理学特征进行描述。(3)对TFE3-VT患者进行生存分析, 描述13例TFE3-VT患者的随访情况, 并与TFE3-non-VT及non-TFE3-VT进行生存情况比较。在与TFE3-non-VT患者进行比较时, 利用Kaplan-Meier法绘制临床分期Ⅰ~Ⅳ期TFE3-RCC患者、TFE3-VT与TFE3-non-VT患者, 以及临床分期Ⅲ期TFE3-VT与TFE3-non-VT亚组患者的PFS及OS曲线, 通过Log-rank检验比较各组患者生存曲线之间的差异是否有统计学意义; 在与non-TFE3-VT患者进行比较时, 采用1 ∶1倾向性评分匹配(propensity score matching, PSM)对两组患者进行配对, 利用Kaplan-Meier法绘制配对后两组患者的PFS及OS曲线, 通过Log-rank检验比较两组患者生存曲线之间的差异是否有统计学意义。所有统计分析均使用R语言(v 4.2.3)进行, 双侧检验P < 0.05为差异有统计学意义。结果: 共纳入45例TFE3-RCC患者, 其中TFE3-VT患者13例, TFE3-non-VT患者32例, 同时纳入non-TFE3-VT患者523例。13例TFE3-VT患者中女性9例(69.2%), 男性4例(30.8%), 平均年龄(37.9±14.4)岁, 平均体重指数(body mass index, BMI)为(22.2±3.5) kg/m2, 中位年龄校正Charlson合并症指数(age-adjusted Charlson comorbidity index, aCCI)为1.0(0.0, 1.0)分, 术前平均肌酐为(75.3±15.9) μmol/L; 7例(53.8%)患者肿瘤位于左肾, 6例(46.2%)位于右肾; 6例(46.2%)患者在术前存在远处转移(M1期), 7例(53.8%)术前未发现远处转移; 合并Mayo 0级VT的患者共7例(53.8%), 合并Mayo Ⅰ级及Mayo Ⅳ级VT的患者各1例(7.7%), 合并Mayo Ⅱ级及Mayo Ⅲ级VT的患者各2例(15.4%); 2例(15.4%)行开放手术, 6例(46.1%)行腹腔镜手术, 5例(38.5%)行机器人辅助腹腔镜手术, 平均手术时长(273±79) min, 平均出血量(722±570) mL; 13例患者肿瘤大体标本的平均最大直径为(10.8±2.4) cm。13例患者均进行了TFE3蛋白免疫组织化学(immunohistochemistry, IHC)染色, 其中7例患者进行了进一步的荧光原位杂交(fluorescence in situ hybridization, FISH)检测并证实为TFE3-RCC。13例患者在随访中有11例(84.6%)出现肿瘤的复发转移, 9例患者(69.2%)死亡, 中位PFS为4个月, 1年PFS率为31%;中位OS为13个月, 1年OS率为54%。在45例TFE3-RCC患者中, 不同临床分期患者的PFS及OS曲线之间差异有统计学意义(P < 0.001);TFE3-VT患者与TFE3-non-VT患者的PFS及OS曲线之间差异有统计学意义(P < 0.001);在临床Ⅲ期患者的亚组分析中, TFE3-VT患者与TFE3-non-VT患者的PFS及OS曲线之间差异仍有统计学意义(P < 0.05)。PSM后, TFE3-VT患者与non-TFE3-VT患者的PFS曲线之间差异有统计学意义(P=0.01), OS曲线之间的差异无统计学意义(P=0.11)。结论: TFE3-VT患者以中青年女性为主, 术前远处转移发生率高; IHC染色TFE3蛋白呈强阳性、FISH检测见红-绿分离信号可明确诊断; TFE3-VT患者与TFE3-non-VT患者相比生存预后较差; TFE3-VT患者较non-TFE3-VT患者更易发生早期进展。
中图分类号:
- R737.11
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
王军卫, 夏丹, 汪朔, 等. 成人Xp11.2/TFE3基因融合相关性肾细胞癌的临床病理特征及预后分析[J]. 中华泌尿外科杂志, 2022, 43 (3): 165- 170.
|
| 7 |
简远熙, 吴俊霖, 杨素萍. MiT家族易位性肾细胞癌的影像学表现[J]. 临床放射学杂志, 2022, 41 (7): 1379- 1383.
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
黄艳, 王玉环, 丁丽红, 等. TFE3重排肾细胞癌16例临床病理特征及预后分析[J]. 诊断病理学杂志, 2024, 31 (8): 732- 736.
|
| 12 |
|
| 13 |
Cheng AV, Wu DJ, Friedman LA, et al. MAPK1IP1L: TFE3-rearranged renal cell carcinoma: A novel fusion adding to the differential diagnosis of oncocytic renal neoplasms [J]. Virchows Arch, 2025, 1(2025-01-25)[2025-01-30]. https://pubmed.ncbi.nlm.nih.gov/39862330.
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| [1] | 郭博达, 陆敏, 王国良, 张洪宪, 刘磊, 侯小飞, 赵磊, 田晓军, 张树栋. 肾透明细胞癌与非透明细胞癌伴静脉癌栓患者的临床病理特征及预后比较[J]. 北京大学学报(医学版), 2025, 57(4): 644-649. |
| [2] | 周泽臻, 葛力源, 张帆, 邓绍晖, 颜野, 张洪宪, 王国良, 刘磊, 黄毅, 张树栋. 病理T3a期肾细胞癌肾部分切除与根治性肾切除的回顾性匹配研究[J]. 北京大学学报(医学版), 2025, 57(4): 704-710. |
| [3] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [4] | 兰东,刘茁,李宇轩,王国良,田晓军,马潞林,张树栋,张洪宪. 根治性肾切除和静脉癌栓取出术大出血的危险因素[J]. 北京大学学报(医学版), 2023, 55(5): 825-832. |
| [5] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
| [6] | 许云屹,苏征征,郑林茂,张孟尼,谭珺娅,杨亚蓝,张梦鑫,徐苗,陈铌,陈雪芹,周桥. 转录通读环状RNA rt-circ-HS促进低氧诱导因子1α表达和肾癌细胞增殖与侵袭[J]. 北京大学学报(医学版), 2023, 55(2): 217-227. |
| [7] | 李东,邸吉廷,熊焰. 程序性细胞死亡1-配体1在不同免疫组织化学染色方法的一致性比较[J]. 北京大学学报(医学版), 2023, 55(2): 339-342. |
| [8] | 张铨,宋海峰,马冰磊,张喆楠,周朝晖,李傲林,刘军,梁磊,朱时雨,张骞. 术前预后营养指数可作为预测非转移性肾细胞癌预后的指标[J]. 北京大学学报(医学版), 2023, 55(1): 149-155. |
| [9] | 博尔术,洪鹏,张宇,邓绍晖,葛力源,陆敏,李楠,马潞林,张树栋. 乳头状肾细胞癌的临床病理特征和预后分析[J]. 北京大学学报(医学版), 2022, 54(4): 615-620. |
| [10] | 周鑫,李文智. 肾细胞癌极致保肾时代的冷思考[J]. 北京大学学报(医学版), 2022, 54(4): 595-598. |
| [11] | 田雨,程晓悦,贺慧颖,王国良,马潞林. 肾细胞癌合并尿路瘤栓的临床病理特征: 6例报道及文献回顾[J]. 北京大学学报(医学版), 2021, 53(5): 928-932. |
| [12] | 韩松辰,黄子雄,刘慧鑫,徐涛. 单侧肾细胞癌根治性切除术后的肾功能代偿[J]. 北京大学学报(医学版), 2021, 53(4): 680-685. |
| [13] | 赵勋,颜野,黄晓娟,董靖晗,刘茁,张洪宪,刘承,马潞林. 癌栓粘连血管壁对非转移性肾细胞癌合并下腔静脉癌栓患者手术及预后的影响[J]. 北京大学学报(医学版), 2021, 53(4): 665-670. |
| [14] | 孙争辉,黄晓娟,董靖晗,刘茁,颜野,刘承,马潞林. 临床T1期肾细胞癌肾窦侵犯的危险因素[J]. 北京大学学报(医学版), 2021, 53(4): 659-664. |
| [15] | 于妍斐,何世明,吴宇财,熊盛炜,沈棋,李妍妍,杨风,何群,李学松. 延胡索酸水合酶缺陷型肾细胞癌的临床病理特征及预后[J]. 北京大学学报(医学版), 2021, 53(4): 640-646. |
|
||