北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (1): 115-119. doi: 10.19723/j.issn.1671-167X.2019.01.021
骨组织工程支架3D打印系统的建立与支架宏微结构精度的可控性评价
李榕1,陈科龙2,王勇1,刘云松1,周永胜1,△( ),孙玉春1,△(
),孙玉春1,△( )
)
- 1. 北京大学口腔医学院·口腔医院,口腔医学数字化研究中心,口腔修复教研室 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室, 北京 100081
2. 北京实诺泰克科技有限公司, 北京 100080
Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision
Rong LI1,Ke-long CHEN2,Yong WANG1,Yun-song LIU1,Yong-sheng ZHOU1,△( ),Yu-chun SUN1,△(
),Yu-chun SUN1,△( )
)
- 1. Center for Digital Dentistry, Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Shinotech Co., Ltd, Beijing 100080, China
摘要:
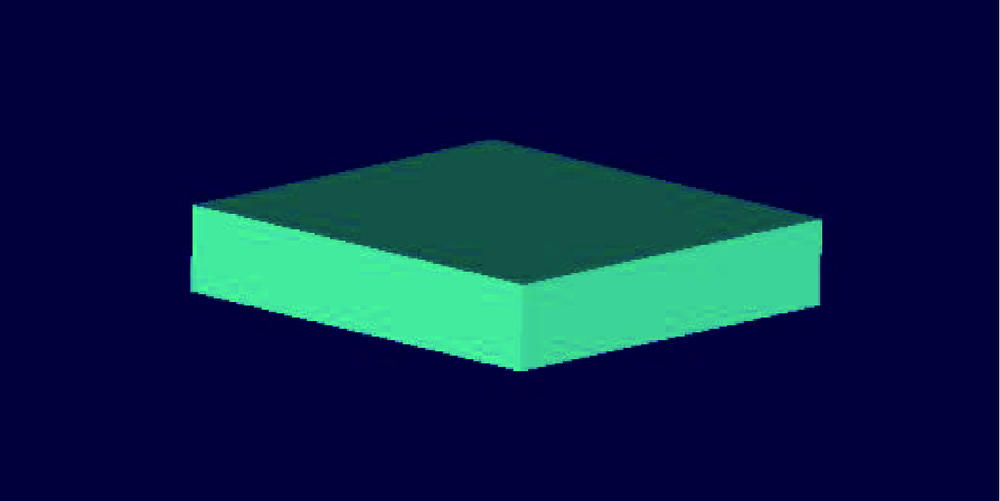
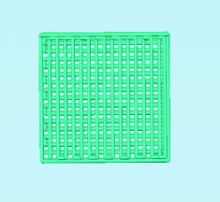
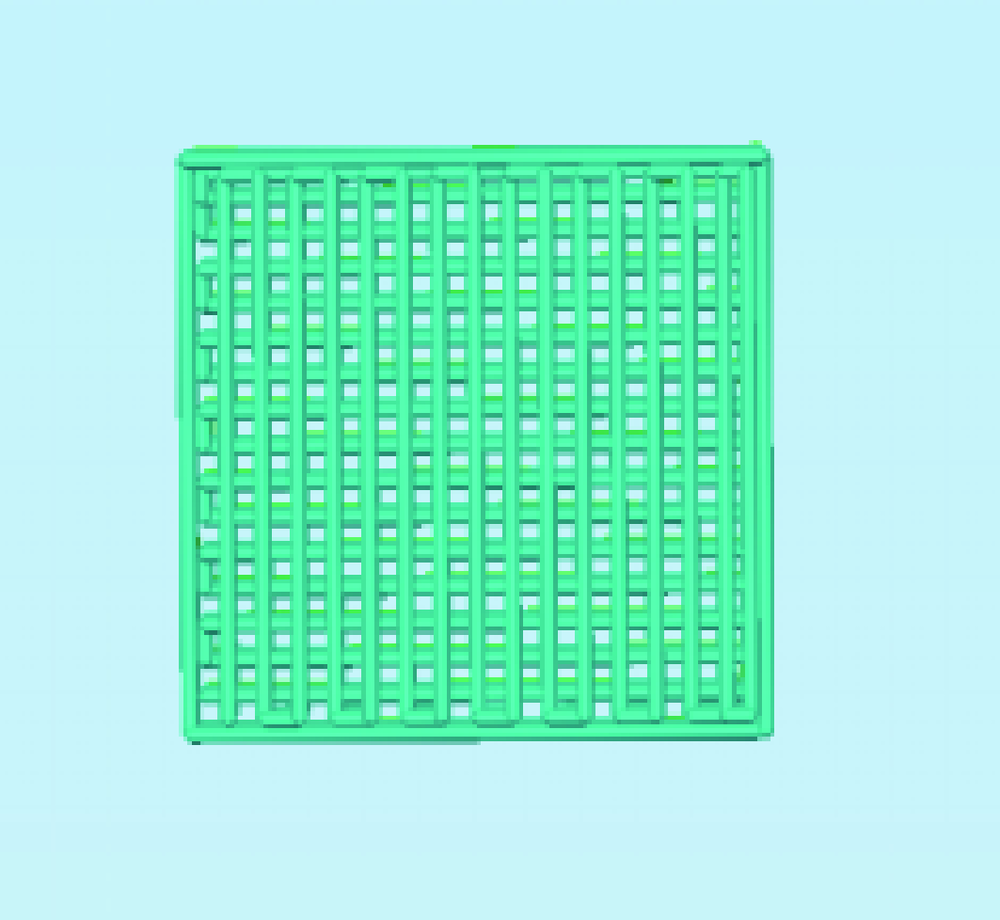
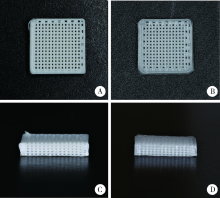
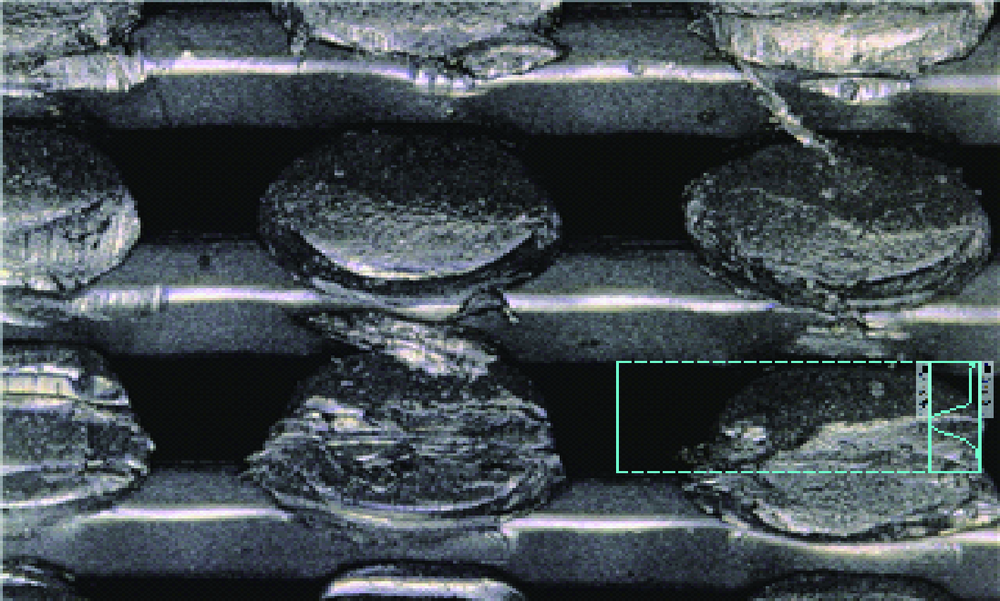
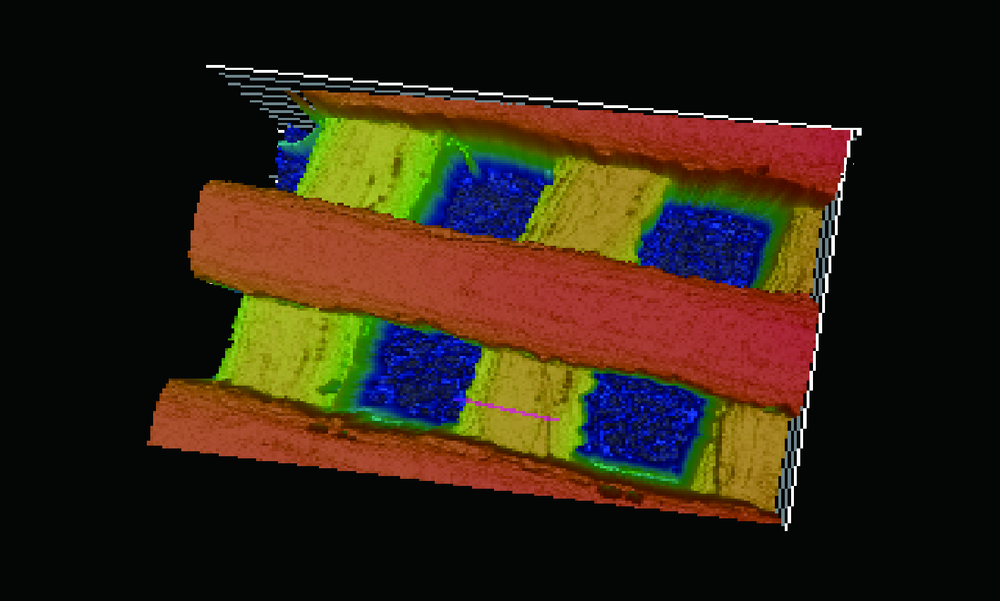
目的:自主研发一种基于熔融沉积成形原理的骨组织工程支架3D打印系统,定量评价其打印聚乳酸(polylactide,PLA)、聚己内酯(polycaprolactone, PCL)制件的宏观和微观结构精度可控性。方法:系统硬件部分为基于三轴步进电机控制的单喷头熔融挤出材料的混元-Ⅰ型生物打印机,喷头直径为0.3 mm,配套打印分层软件生成打印控制代码为Gcode格式文件。用Imageware设计长×宽×高为10 mm×10 mm×2 mm的长方体,保存成STL文件。将文件导入配套打印分层软件并设定长方体内部为均匀分布的长方体孔隙结构,打印层厚0.2 mm,生成Gcode代码并用混元-Ⅰ型生物打印机分别打印PLA和PCL制件,每种材料重复打印10次。打印完成并充分自然冷却后取下,获得PLA、PCL打印制件(10个×2组)。用游标卡尺测量每个制件的宏观尺寸,每组任意选取3个制件用激光三维形貌测量显微镜扫描并测量每个制件层间重叠和无层间重叠区域的孔隙尺寸与实体支撑梁的直径。结果:所建立系统打印的PLA、PCL制件孔隙规则且相互贯通,宏观尺寸分别为PLA:长 9.950(0.020) mm,宽 9.950(0.003) mm,高 1.970(0.023) mm;PCL:长 9.845(0.025) mm,宽 9.845(0.045) mm,高 1.950(0.043) mm。内部结构PLA、PCL层间重叠部分支撑梁直径稍有增粗,前者较明显。各测量值中PLA层间重叠区域孔隙(274.09±8.35) μm与设计值差值最大,为26.91 μm。结论:应用自主研发的组织工程支架3D打印系统可完成PLA、PCL多孔支架的打印,该系统对宏观、微观结构的可控性满足研究应用需求。
中图分类号:
- R78
| [1] |
Langer R, Vacanti JP . Tissue engineering[J]. Science, 1993,260(5110):920-926.
doi: 10.1126/science.8493529 |
| [2] |
Kneser U, Schaefer DJ, Polykandriotis E , et al. Tissue engineering of bone: the reconstructive surgeon’s point of view[J]. J Cell Mol Med, 2006,10(1):7-19.
doi: 10.1111/jcmm.2006.10.issue-1 |
| [3] |
Hutmacher DW . Scaffolds in tissue engineering bone and cartilage[J]. Biomaterials, 2000,21(24):2529-2543.
doi: 10.1016/S0142-9612(00)00121-6 pmid: 11071603 |
| [4] |
Jia A, Joanne EM, Ratima S , et al. Design and 3D printing of scaffolds and tissues[J]. Engineering, 2015,1(2):261-268.
doi: 10.15302/J-ENG-2015061 |
| [5] |
Peltola SM, Melchels FP, Grijpma DW , et al. A review of rapid prototyping techniques for tissue engineering purposes[J]. Ann Med, 2008,40(4):268-280.
doi: 10.1080/07853890701881788 |
| [6] |
Rezwan K, Chen QZ, Blaker JJ , et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering[J]. Biomaterials, 2006,27(18):3413-3431.
doi: 10.1016/j.biomaterials.2006.01.039 pmid: 16504284 |
| [7] |
Hulbert SF, Young FA, Mathews RS , et al. Potential of ceramic materials as permanently implantable skeletal prostheses[J]. J Biomed Mater Res, 1970,4(3):433-456.
doi: 10.1002/jbm.820040309 pmid: 5469185 |
| [8] |
Kuboki Y, Jin Q, Takita H . Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis[J]. J Bone Joint Surg Am, 2001,83(A Suppl 1Pt 2):S105-115.
doi: 10.1054/arth.2001.9052 pmid: 11314788 |
| [9] |
Tarawneh AM, Wettergreen M, Liebschner MAK . Computer-aided tissue engineering: benefiting from the control over scaffold micro-architecture[J]. Methods Mol Biol, 2012,868:1-25.
doi: 10.1007/978-1-61779-764-4 |
| [10] |
Campos Marin A, Lacroix D . The inter-sample structural variability of regular tissue-engineered scaffolds significantly affects the micromechanical local cell environment[J]. Interface Focus, 2015,5(2):20140097.
doi: 10.1098/rsfs.2014.0097 pmid: 4342953 |
| [11] |
Yu W, Hong Q, Hu G , et al. A Microfluidic-based multi-shear device for investigating the effects of low fluid-induced stresses on osteoblasts[J]. PLoS One, 2014,9(2):e89966.
doi: 10.1371/journal.pone.0089966 pmid: 24587156 |
| [12] |
Tarafder S, Balla VK, Davies NM , et al. Microwave sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering[J]. J Tissue Eng Regen Med, 2013,7(8):631-641.
doi: 10.1002/term.555 pmid: 22396130 |
| [13] |
李树袆, 周苗, 赖毓霄 , 等. 三维打印聚乳酸-羟基乙酸/磷酸三钙骨修复支架的生物学评价[J]. 中华口腔医学杂志, 2016,51(11):661-666.
doi: 10.3760/cma.j.issn.1002-0098.2016.11.005 |
| [14] |
Bergsma JE, Bruijn WCD, Rozema FR , et al. Late degradation tissue response to poly(l-lactide) bone plates and screws[J]. Biomaterials, 1995,16(1):25-31.
doi: 10.1016/0142-9612(95)91092-D pmid: 7718688 |
| [15] |
Böstman O, Hirvensalo E, Mäkinen J , et al. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers[J]. J Bone Joint Surg Br, 1990,72(4):592-596.
doi: 10.1016/0020-1383(90)90021-L pmid: 2199452 |
| [16] |
Woodruff MA, Hutmacher DW . The return of a forgotten polymer & mdash; polycaprolactone in the 21st century[J]. Prog Polym Sci, 2010,35(10):1217-1256.
doi: 10.1016/j.progpolymsci.2010.04.002 |
| [17] |
Xu N, Ye X, Wei D , et al. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair[J]. ACS Appl Mater Interfaces, 2014,6(17):14952-14963.
doi: 10.1021/am502716t pmid: 25133309 |
| [18] |
Li Y, Wu ZG, Li XK , et al. A polycaprolactone-tricalcium phosphate composite scaffold as an autograft-free spinal fusion cage in a sheep model[J]. Biomaterials, 2014,35(22):5647-5659.
doi: 10.1016/j.biomaterials.2014.03.075 pmid: 24743032 |
| [1] | 王明瑞, 王起, 胡浩, 赖金惠, 唐鑫伟, 万春艳, 许克新, 徐涛. 覆膜金属输尿管支架治疗盆腔脂肪增多症所致肾积水的疗效[J]. 北京大学学报(医学版), 2024, 56(5): 919-922. |
| [2] | 杨文博,余磊,张维宇,徐涛,王强. 带线输尿管支架自排技术在肾移植受者中的效果及安全性[J]. 北京大学学报(医学版), 2024, 56(4): 656-660. |
| [3] | 赖金惠,王起,姬家祥,王明瑞,唐鑫伟,许克新,徐涛,胡浩. 新型冠状病毒肺炎疫情期间延迟拔除输尿管支架对泌尿系结石术后患者生活质量和心理状态的影响[J]. 北京大学学报(医学版), 2023, 55(5): 857-864. |
| [4] | 韩金涛,张宇翔,贾子昌,姜除寒,刘恋,栾景源,梁飞,赵彦清. Neuroform Atlas支架辅助弹簧圈栓塞未破裂性颅内宽颈动脉瘤[J]. 北京大学学报(医学版), 2023, 55(1): 139-143. |
| [5] | 李雨柯,王梅,唐琳,刘玉华,陈晓颖. 不同pH值对脱细胞小肠黏膜下层海绵支架螯合锶离子的影响[J]. 北京大学学报(医学版), 2023, 55(1): 44-51. |
| [6] | 张春龙,王明瑞,王起,许克新,徐涛,胡浩. 覆膜金属输尿管支架维持性治疗输尿管镜碎石术后难治性输尿管狭窄的远期疗效评价[J]. 北京大学学报(医学版), 2022, 54(4): 674-679. |
| [7] | 邓艺,张一,李博文,王梅,唐琳,刘玉华. 不同交联剂处理对脱细胞小肠黏膜下层多孔支架的影响[J]. 北京大学学报(医学版), 2022, 54(3): 557-564. |
| [8] | 朱正达,高岩,何汶秀,方鑫,刘洋,魏攀,闫志敏,华红. 红色诺卡氏菌细胞壁骨架治疗糜烂型口腔扁平苔藓的疗效及安全性[J]. 北京大学学报(医学版), 2021, 53(5): 964-969. |
| [9] | 庄金满,李天润,李选,栾景源,王昌明,冯琦琛,韩金涛. Rotarex 旋切导管在下肢动脉硬化闭塞症支架内再狭窄中的应用[J]. 北京大学学报(医学版), 2021, 53(4): 740-743. |
| [10] | 王梅, 李博文, 王思雯, 刘玉华. 猪小肠黏膜下层海绵的制备及促成骨作用[J]. 北京大学学报(医学版), 2020, 52(5): 952-958. |
| [11] | 董文敏,王明瑞,胡浩,王起,许克新,徐涛. Allium覆膜金属输尿管支架长期留置治疗输尿管-回肠吻合口狭窄的初期临床经验及随访结果[J]. 北京大学学报(医学版), 2020, 52(4): 637-641. |
| [12] | 曹春玲,杨聪翀,屈小中,韩冰,王晓燕. 可注射羟乙基壳聚糖基水凝胶理化性能及其对人牙髓细胞增殖和成牙本质向分化的作用[J]. 北京大学学报(医学版), 2020, 52(1): 10-17. |
| [13] | 贾子昌,李选,郑梅,栾景源,王昌明,韩金涛. 复合手术治疗无残端的症状性长段颈内动脉慢性闭塞[J]. 北京大学学报(医学版), 2020, 52(1): 177-180. |
| [14] | 赵海燕,樊东升,韩金涛. 重度颈内动脉狭窄伴未破裂动脉瘤的治疗策略[J]. 北京大学学报(医学版), 2019, 51(5): 829-834. |
| [15] | 贾子昌,卞焕菊,李选,栾景源,王昌明,刘启佳,韩金涛. Neuroform EZ支架在治疗复杂症状性颅内动脉重度狭窄中的应用[J]. 北京大学学报(医学版), 2019, 51(5): 835-839. |
|
||