北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (2): 270-275. doi: 10.19723/j.issn.1671-167X.2023.02.010
JAK/STAT信号通路在卵巢高级别浆液性癌中的激活及预后意义
- 1. 北京大学第三医院病理科, 北京 100191
2. 北京大学基础医学院病理学系, 北京 100191
Activation of JAK/STAT in ovarian high-grade serous cancers and its prognostic significance
Jing YANG1,Juan DU1,2,Yu-xiang WANG1,2,Cong-rong LIU1,2,*( )
)
- 1. Department of Pathology, Peking University Third Hospital, Beijing 100191, China
2. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191, China
摘要:
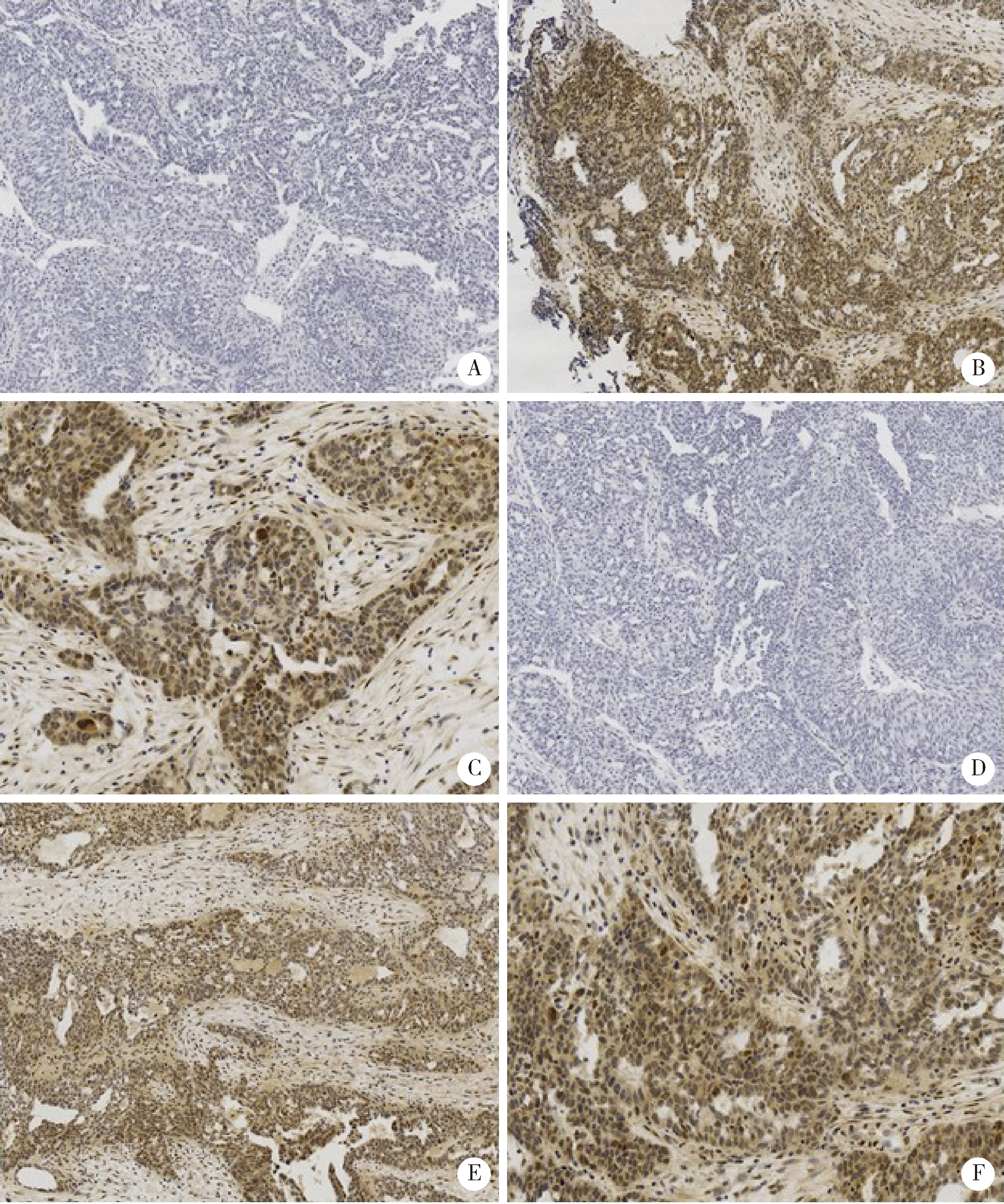
目的: Janus激酶(Janus kinase, JAK)、信号转导和转录活化因子(signal transducers and activators of transcription, STAT)构成的JAK/STAT信号通路的活化与卵巢高级别浆液性癌(high-grade serous carcinoma, HGSC)的预后和靶向治疗密切相关。本研究力求通过简单易行的检测手段, 评估JAK/STAT信号通路的活化在卵巢HGSC中的预后意义。方法: 运用免疫组织化学染色方法, 对73例卵巢HGSC的病理切片进行磷酸化STAT3(pSTAT3)和磷酸化STAT5(pSTAT5)染色, 并对二者的染色强度及染色范围进行定量评估。以此为标准, 将HGSC病例分为pSTAT3低/高表达组以及pSTAT5低/高表达组, 并对不同分组的患者预后情况进行分析, 探究pSTAT3和pSTAT5的表达与HGSC预后的关系。结果: 部分卵巢HGSC存在pSTAT3和pSTAT5蛋白的高表达, 且二者的表达与HGSC的预后相关。pSTAT3和pSTAT5蛋白的表达水平在预后较好组(生存期≥3年)和预后较差组(生存期 < 3年)中的差异有统计学意义, 伴有pSTAT3高表达、或pSTAT5高表达、或pSTAT3和pSTAT5皆高表达的HGSC病例预后更差, 表现为疾病无进展生存期以及总生存期均显著低于对应的低表达组(P < 0.001)。结论: 通过pSTAT3、pSTAT5蛋白的免疫组织化学染色, 可以实现对卵巢HGSC患者进行风险评估, 可协助判断预后同时筛选高危人群, 为STAT抑制剂及抗血管生成药物的适用人群提供有益的参考指标。
中图分类号:
- R737.31
| 1 |
Roeser JC , Leach SD , McAllister F . Emerging strategies for cancer immunoprevention[J]. Oncogene, 2015, 34 (50): 6029- 6039.
doi: 10.1038/onc.2015.98 |
| 2 |
Frank DA . STAT3 as a central mediator of neoplastic cellular transformation[J]. Cancer Lett, 2007, 251 (2): 199- 210.
doi: 10.1016/j.canlet.2006.10.017 |
| 3 |
Suh YA , Jo SY , Lee HY . Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells[J]. Int J Oncol, 2015, 46 (3): 1405- 1411.
doi: 10.3892/ijo.2014.2808 |
| 4 |
Chen H , Ye D , Xie X , et al. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma[J]. Gynecol Oncol, 2004, 94 (3): 630- 635.
doi: 10.1016/j.ygyno.2004.05.056 |
| 5 |
Saini U , Naidu S , ElNaggar AC , et al. Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: A potential therapeutic target[J]. Oncogene, 2017, 36 (2): 168- 181.
doi: 10.1038/onc.2016.197 |
| 6 | Perez RP , Godwin AK , Hamilton TC , et al. Ovarian cancer biology[J]. Semin Oncol, 1991, 18 (3): 186- 204. |
| 7 |
Zhang H , Liu T , Zhang Z , et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer[J]. Cell, 2016, 166 (3): 755- 765.
doi: 10.1016/j.cell.2016.05.069 |
| 8 |
Szubert S , Moszynski R , Szpurek D , et al. The expression of platelet-derived growth factor receptors (PDGFRs) and their correlation with overall survival of patients with ovarian cancer[J]. Ginekol Pol, 2019, 90 (5): 242- 249.
doi: 10.5603/GP.a2019.0045 |
| 9 |
Gadducci A , Lanfredini N , Sergiampietri C . Antiangiogenic agents in gynecological cancer: State of art and perspectives of clinical research[J]. Crit Rev Oncol Hematol, 2015, 96 (1): 113- 128.
doi: 10.1016/j.critrevonc.2015.05.009 |
| 10 |
Aravantinos G , Pectasides D . Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: A systematic review[J]. J Ovarian Res, 2014, 7, 57.
doi: 10.1186/1757-2215-7-57 |
| [1] | 葛晓东,李妹玲,文曦琳,李易,邓小林,吴晓凤,文明,李少林. 超顺磁性氧化铁短发夹RNA双功能分子探针外转染卵巢癌细胞的最佳浓度[J]. 北京大学学报(医学版), 2015, 47(5): 754-760. |
| [2] | 马瑞琼,程洪艳,叶雪,陈军,崔恒,魏丽惠,昌晓红. 肿瘤坏死因子受体相关蛋白1在上皮性卵巢癌组织中的表达及意义[J]. 北京大学学报(医学版), 2014, 46(1): 120-124. |
| [3] | 杨静, 张旗, 董建强, 昌晓红, 何湘君. 高迁移率蛋白A2在浆液性卵巢癌中高表达及其与let-7家族microRNA的关系[J]. 北京大学学报(医学版), 2012, 44(5): 749-754. |
| [4] | 王晓慧, 雷军强, 彭芝兰, 杨永秀. △Np63基因表达与卵巢癌生物学行为的关系[J]. 北京大学学报(医学版), 2011, 43(2): 304-306. |
| [5] | 马丽萍, 李娜, 何湘君, 张旗. miR-449b和miR-34c对卵巢癌细胞SKOV3-ipl周期相关蛋白的下调及细胞周期阻滞作用[J]. 北京大学学报(医学版), 2011, 43(1): 129-133. |
| [6] | 赵旸, 王悦, 沈丹华, 宋荣娜, 许琦, 李艺, 崔恒, 唐军, 魏丽惠. 卵巢交界性肿瘤及Ⅰ期上皮性卵巢癌143例临床分析[J]. 北京大学学报(医学版), 2011, 43(1): 123-128. |
| [7] | 张超, 崔恒, 李小平, 叶雪, 付天云, 冯捷, 昌晓红, 魏丽惠. 卵巢癌抗抗独特型单克隆抗体3D12的制备及初步研究[J]. 北京大学学报(医学版), 2008, 40(2): 170-173. |
| [8] | 陆红霞, 徐丛剑, 李斌, 康玉, 黄倩, 李立民, 陈庆华. 紫杉醇纳米粒腹腔给药对大鼠卵巢癌的抑制作用及淋巴靶向性[J]. 北京大学学报(医学版), 2006, 38(5): 483-486. |
| [9] | 易晓芳, 范士明, 姚明, 丰有吉. 腹腔与静脉应用托泊替坎治疗人卵巢癌细胞株SKOV3裸小鼠网膜移植瘤的疗效比较[J]. 北京大学学报(医学版), 2006, 38(1): 88-91. |
| [10] | 卢媛, 刘惜时, 王跃祥, 宋后燕, Nanbert ZHONG. 上皮性卵巢癌中微卫星不稳定的研究[J]. 北京大学学报(医学版), 2006, 38(1): 62-65. |
| [11] | 昌晓红, 崔恒, 冯捷, 杨文兰, 李艺, 付天云, 叶雪, 祝洪澜, 程洪艳, 成夜霞, 郭惠方. 卵巢癌6B11抗独特型微抗体诱导抗肿瘤免疫应答的体外实验[J]. 北京大学学报(医学版), 2005, 37(5): 480-484. |
| [12] | 袁碧波, 糜若然, 韩忠朝, 蔡英林, 周毓玲, 马月霞. hTERT基因反义cDNA转染对卵巢癌细胞系SKOV3生物学行为的影响[J]. 北京大学学报(医学版), 2002, 34(6): 680-683. |
| [13] | 崔恒, 冯捷, 钱和年. 抗独特型卵巢癌疫苗的研究[J]. 北京大学学报(医学版), 2002, 34(5): 570-573. |
| [14] | 刘广芝, 冯捷, 叶雪, 付天云, 成夜霞. 人卵巢上皮癌细胞原代培养及细胞系BUPH:OVSC-1的建立[J]. 北京大学学报(医学版), 2002, 34(3): 271-275. |
| [15] | 温宏武. 雌激素受体β mRNA在卵巢上皮性癌中的表达[J]. 北京大学学报(医学版), 2001, 33(5): 424-426. |
|
||