北京大学学报(医学版) ›› 2025, Vol. 57 ›› Issue (3): 569-577. doi: 10.19723/j.issn.1671-167X.2025.03.022
铁死亡相关长链非编码核糖核酸预测放射治疗后非小细胞肺癌患者的临床结局
- 1. 北京大学医学部医学技术研究院, 北京 100191
2. 北京大学第三医院医学创新研究院基础医学研究中心, 北京 100191
3. 北京大学第三医院放射肿瘤科, 北京 100191
Ferroptosis-related long non-coding RNA to predict the clinical outcome of non-small cell lung cancer after radiotherapy
Qiushi XU1, Tong LIU2, Junjie WANG3,*( )
)
- 1. Institute of Medical Technology, Peking University Health Science Center, Beijing 100191, China
2. Center of Basic Medical Research, Institute of Medical Innovation and Research, Peking University Third Hospital, Beijing 100191, China
3. Department of Radiation Oncology, Peking University Third Hospital, Beijing 100191, China
摘要:
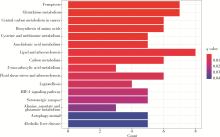
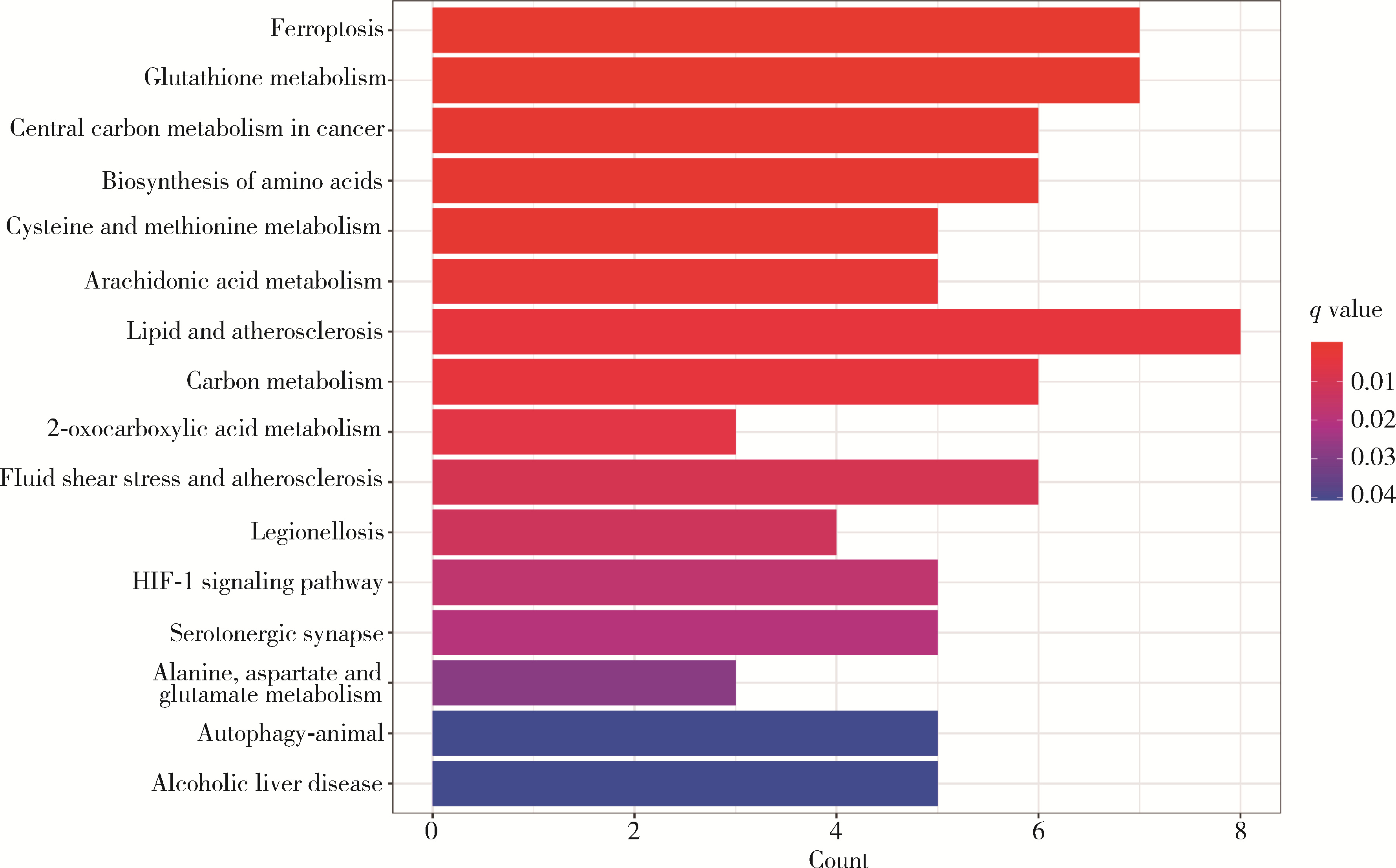
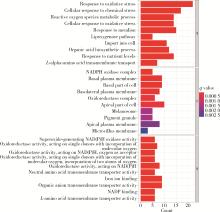
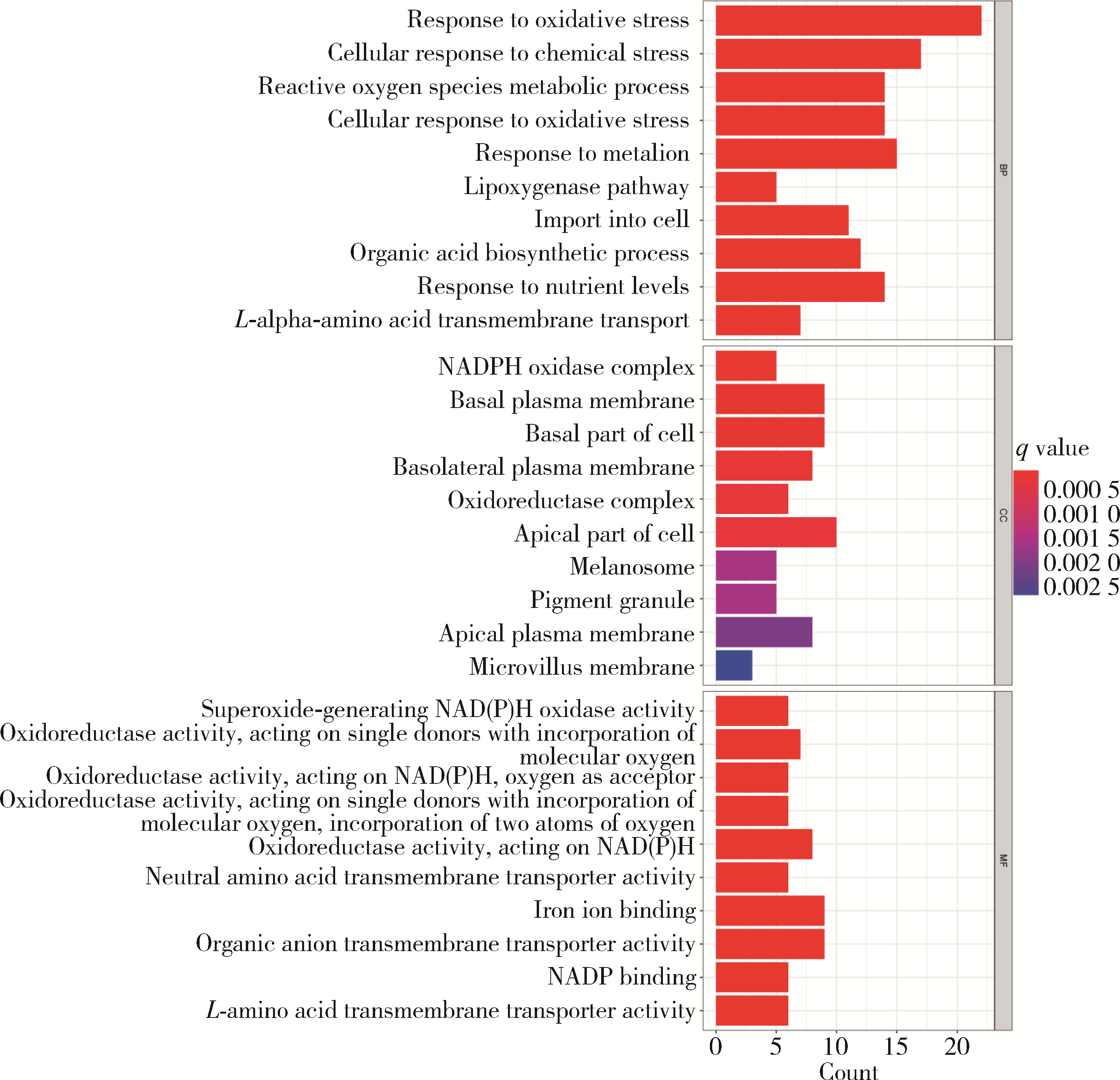
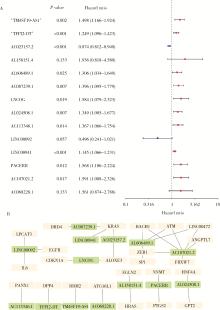
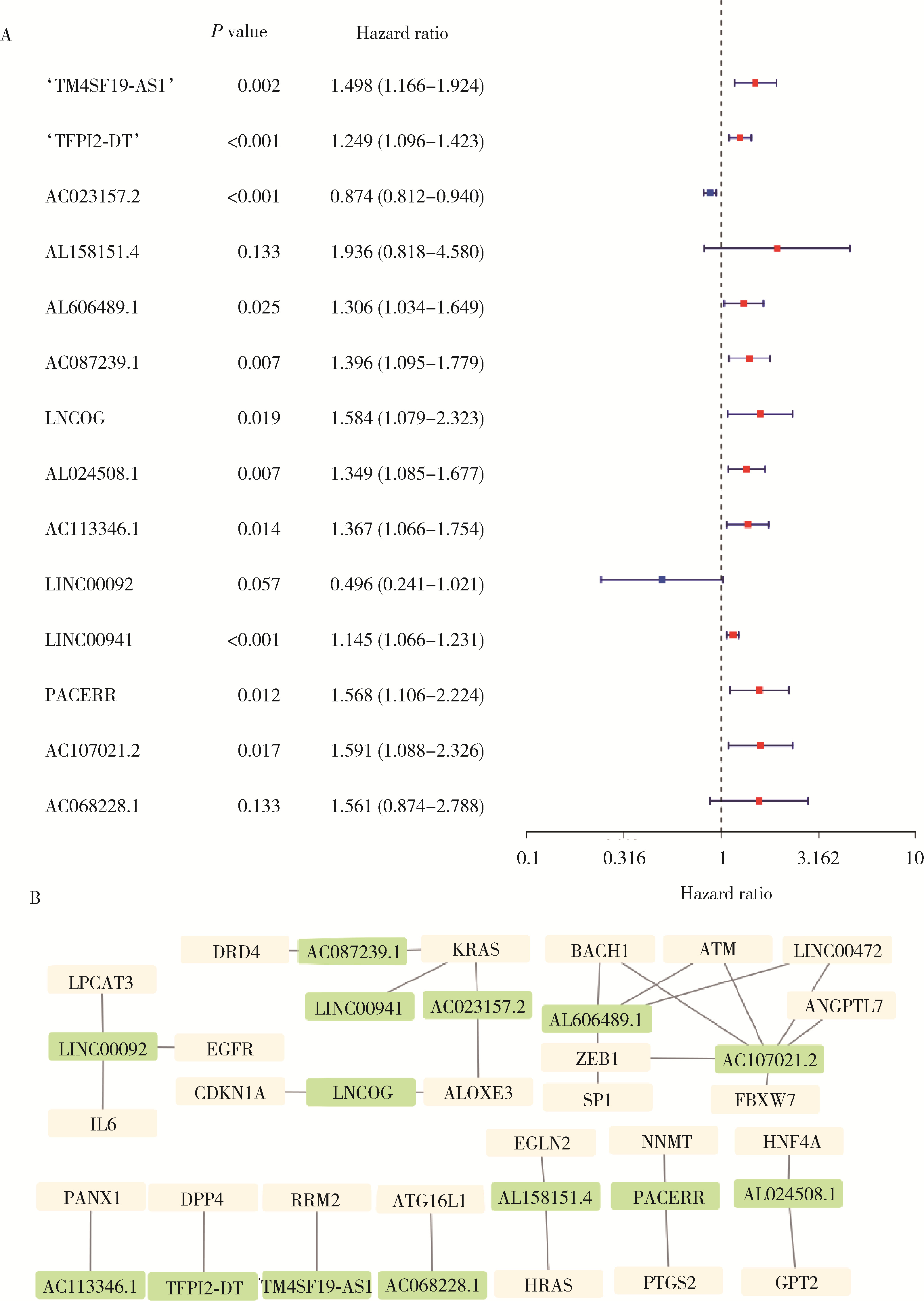
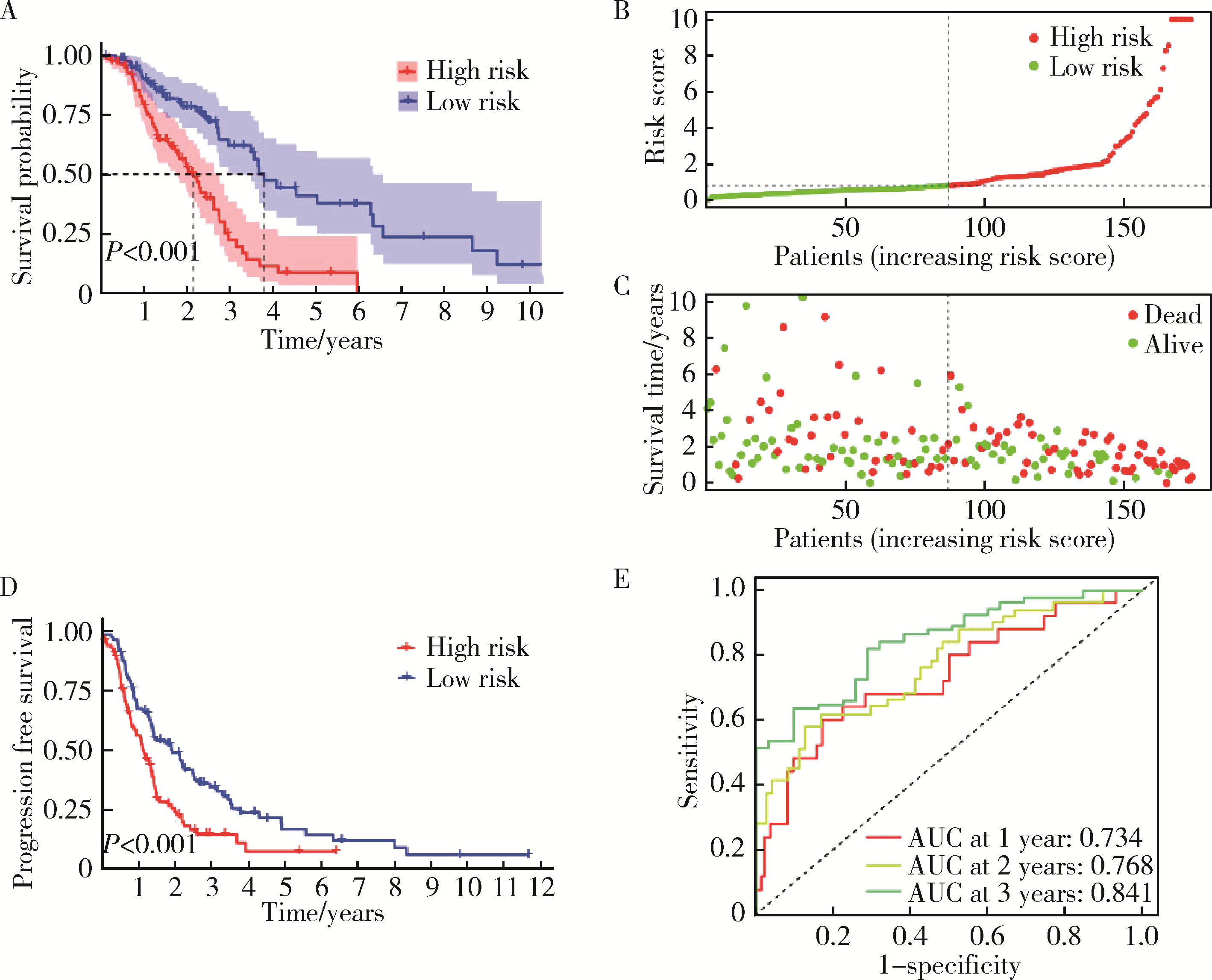
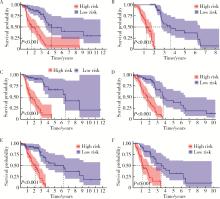
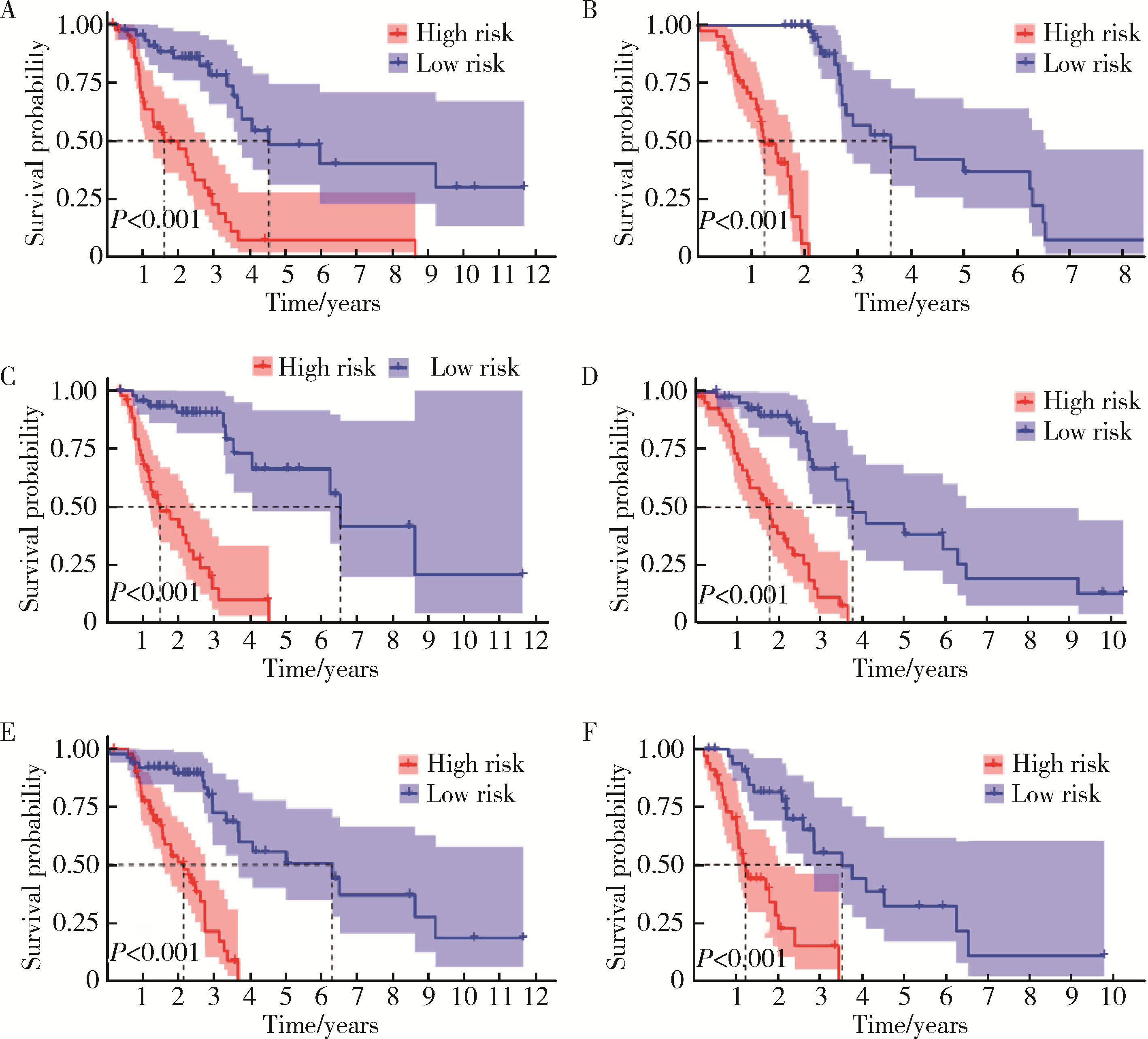
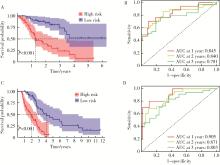
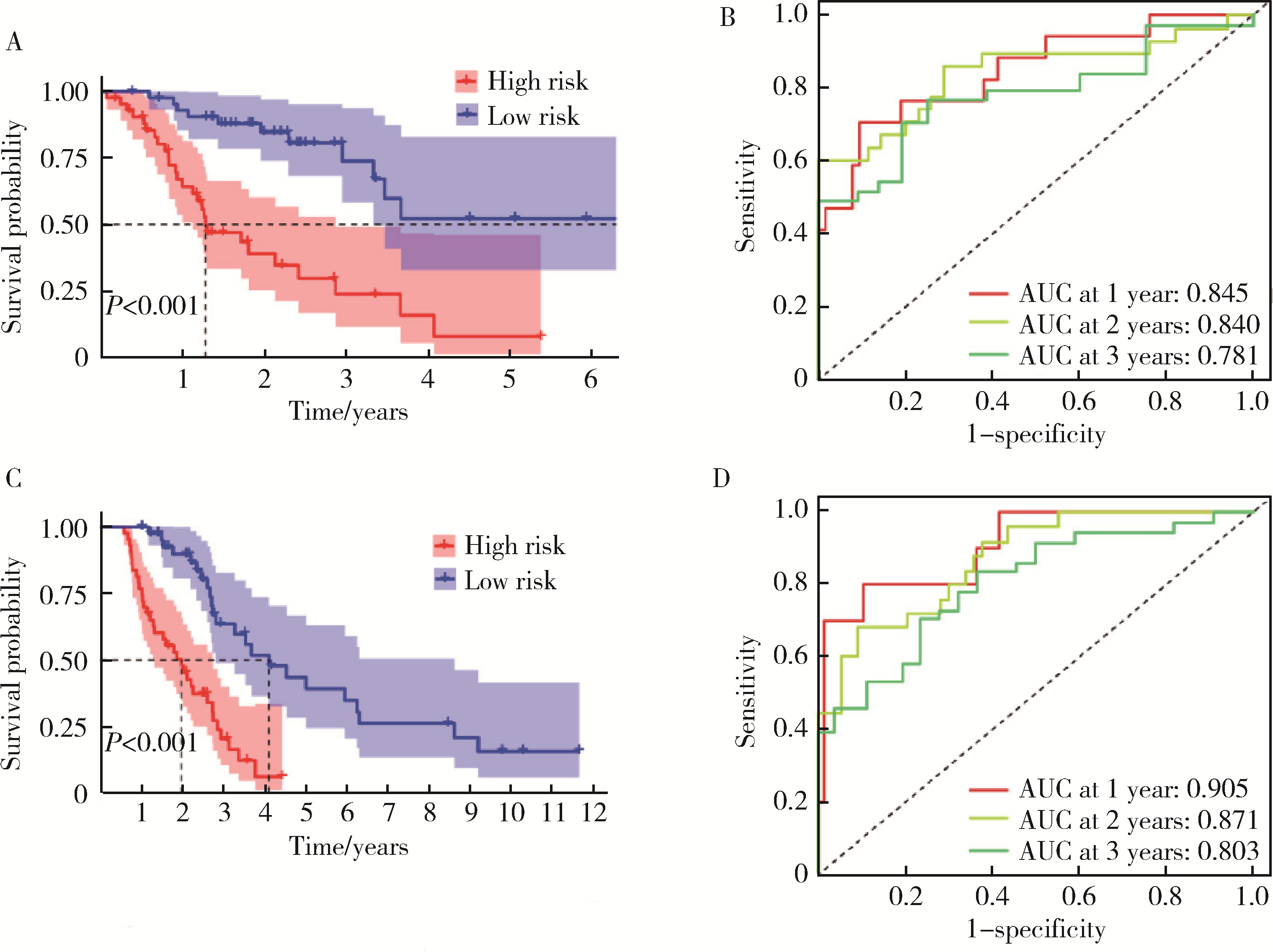
目的: 构建基于铁死亡的相关长链非编码核糖核酸(long non-coding RNA, lncRNA)模型,预测放射治疗(放疗)后非小细胞肺癌患者的预后。方法: 从癌症基因组图谱数据库(the cancer genome atlas,TCGA)下载标准化原发瘤和正常组织转录组数据,以及相应的临床信息数据,进行单变量和多变量Cox回归模型分析,构建与铁死亡相关的lncRNA高、低风险组预测模型,使用数据包预测患者的生存率和无进展生存期,验证模型在高、低风险组中的差异。结果: 铁死亡相关的差异性表达基因主要富集于铁死亡、谷胱甘肽代谢、脂质和动脉粥样硬化信号通路及氧化应激、活性氧的代谢过程;用14个与铁死亡相关的lncRNA构建一个预后模型,数据分析表明铁死亡相关的lncRNA可以独立预测放疗后非小细胞肺癌患者的预后;以年龄、性别、分期作为临床病理学变量,可预测出放疗后非小细胞肺癌高风险组预后较差。结论: 风险模型能够独立预测放疗后非小细胞肺癌患者的预后,可为铁死亡相关lncRNA在放疗后非小细胞肺癌中预后预测提供依据,并为非小细胞肺癌患者放疗联合铁死亡治疗提供临床治疗指导。
中图分类号:
- R34
| 1 |
doi: 10.3322/caac.21590 |
| 2 |
doi: 10.3390/ijms22168661 |
| 3 |
doi: 10.1097/CCO.0000000000000703 |
| 4 |
|
| 5 |
doi: 10.1056/NEJMra1608986 |
| 6 |
doi: 10.1158/1078-0432.CCR-12-0298 |
| 7 |
|
| 8 |
doi: 10.1016/j.cell.2012.03.042 |
| 9 |
doi: 10.1038/s41422-019-0164-5 |
| 10 |
doi: 10.3389/fcell.2020.586578 |
| 11 |
doi: 10.1016/j.tcb.2015.10.014 |
| 12 |
doi: 10.1158/2159-8290.CD-19-0338 |
| 13 |
doi: 10.1038/s41422-019-0263-3 |
| 14 |
doi: 10.1038/onc.2011.621 |
| 15 |
doi: 10.1016/j.biocel.2013.05.030 |
| 16 |
doi: 10.1186/s12967-015-0556-3 |
| 17 |
doi: 10.1038/cddis.2015.30 |
| 18 |
doi: 10.1158/1535-7163.MCT-14-0492 |
| 19 |
doi: 10.1136/gutjnl-2013-305266 |
| 20 |
|
| 21 |
doi: 10.1039/D0NR08757B |
| 22 |
doi: 10.1158/0008-5472.CAN-20-3477 |
| 23 |
|
| 24 |
doi: 10.3389/fcell.2021.675555 |
| 25 |
doi: 10.1021/acschembio.9b00939 |
| 26 |
doi: 10.1186/1756-0500-6-518 |
| 27 |
|
| 28 |
|
| 29 |
doi: 10.1038/s41598-021-83244-7 |
| 30 |
doi: 10.1038/nature08975 |
| 31 |
doi: 10.1371/journal.pone.0000845 |
| 32 |
|
| 33 |
doi: 10.1016/j.tranon.2022.101356 |
| 34 |
doi: 10.1016/j.molcel.2010.03.021 |
| 35 |
doi: 10.1186/s13045-019-0773-y |
| 36 |
doi: 10.1136/jitc-2019-000110 |
| 37 |
doi: 10.1038/s41418-019-0304-y |
| 38 |
doi: 10.1186/s12935-021-02027-2 |
| [1] | 黄万伟, 沙显燊, 张艺宝, 伍国豪, 骆峰, 陈智慧, 叶东明, 李学松, 赖彩永. 完全3D腹腔镜回肠代双侧输尿管联合膀胱扩大术修复放射治疗后双侧输尿管狭窄并膀胱挛缩[J]. 北京大学学报(医学版), 2025, 57(4): 789-795. |
| [2] | 赵柯林, 夏雪, 史乃旭, 周韩, 盖婧雯, 李萍. 铁死亡标志物4-HNE在系统性硬化症细胞模型中的表达及意义[J]. 北京大学学报(医学版), 2024, 56(6): 950-955. |
| [3] | 田素青, 孙海涛, 赵田地, 王巍. 6D治疗床辅助影像引导下放射治疗头颈部肿瘤摆位误差分析[J]. 北京大学学报(医学版), 2024, 56(6): 1097-1100. |
| [4] | 彭圣嘉,祁雨,孙丽杰,李丹,王新宇,韩江莉,陈宝霞,张媛. 传入压力反射衰竭合并低钠血症1例[J]. 北京大学学报(医学版), 2024, 56(2): 357-361. |
| [5] | 姜海红,李小凡,王建六. 宫颈癌慢性放射性肠炎与肠道微生物的关系[J]. 北京大学学报(医学版), 2023, 55(4): 619-624. |
| [6] | 李东,邸吉廷,熊焰. 程序性细胞死亡1-配体1在不同免疫组织化学染色方法的一致性比较[J]. 北京大学学报(医学版), 2023, 55(2): 339-342. |
| [7] | 朱巧,任翠,张艳,李美娇,王晓华. 能谱CT诊断非小细胞肺癌纵隔淋巴结转移的应用价值[J]. 北京大学学报(医学版), 2020, 52(4): 730-737. |
| [8] | 王皓,姜树坤,彭冉,黄毅,王明清,王俊杰,刘承,张帆,马潞林. 个体化尿量控制提高泌尿肿瘤放疗期间膀胱稳定性[J]. 北京大学学报(医学版), 2020, 52(4): 688-691. |
| [9] | 欧阳雨晴,倪莲芳,刘新民. 恶性孤立性肺结节患者预后因素分析[J]. 北京大学学报(医学版), 2020, 52(1): 158-162. |
| [10] | 张春凤,刘云,陆敏,杜晓娟. hUTP14a在非小细胞肺癌组织中的表达[J]. 北京大学学报(医学版), 2019, 51(1): 145-150. |
| [11] | 郭福新,姜玉良,吉喆,彭冉,孙海涛,王俊杰. 3D打印非共面模板辅助CT引导125Ⅰ粒子植入治疗锁骨上复发转移癌的剂量学研究[J]. 北京大学学报(医学版), 2017, 49(3): 506-511. |
| [12] | 王庆国,李晓梅,张敏,李航,温冰,李洪振,高献书. 107例瘢痕疙瘩术后两种分割剂量放疗疗效分析[J]. 北京大学学报(医学版), 2014, 46(1): 169-172. |
| [13] | 武金玉, 陈敏华, 严昆, 张晖, 王惠莉, 霍苓, 杨薇, 宋一平. 超声引导射频消融术应用于治疗肝转移癌[J]. 北京大学学报(医学版), 2001, 33(5): 449-451. |
|
||