北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (2): 269-274. doi: 10.19723/j.issn.1671-167X.2020.02.012
绿原酸对高脂饲料诱导的肥胖大鼠糖耐量及其曲线特征的影响
- 北京大学第三医院运动医学研究所,北京 100191
Effects of chlorogenic acid on glucose tolerance and its curve characteristics in high-fat diet-induced obesity rats
Cheng-cheng GUO,Xiao-yuan ZHANG,Ying-xiang YU,Lan XIE,Cui-qing CHANG( )
)
- Institute of Sports Medicine, Peking University Third Hospital, Beijing 100191, China
摘要:
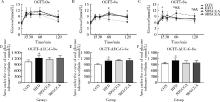
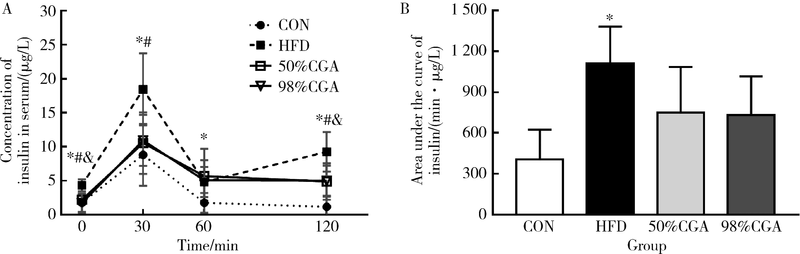
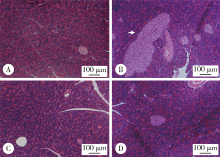
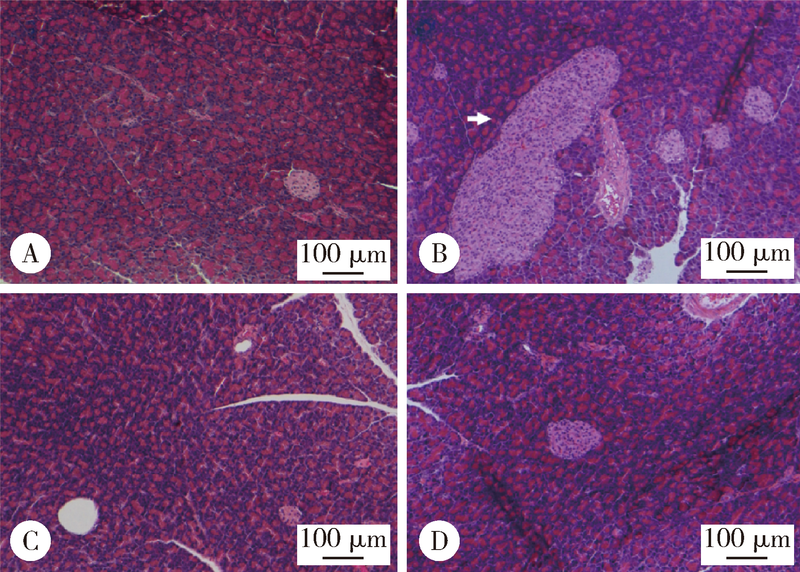
目的 观察绿原酸(chlorogenic acid,CGA)对高脂饲料诱导的肥胖(diet-induced-obesity,DIO)大鼠糖耐量及其曲线特征的作用, 为开发利用CGA早期预防和延缓糖尿病的发生提供依据.方法: 46只雄性Sprague-Dawley(SD)大鼠随机选择8只作为普通饲料组(normal control group,CON), 其余大鼠饲喂高脂饲料.4周后按标准筛选高脂诱导的肥胖大鼠24只并随机分为高脂饲料组(high fat diet group,HFD),50%(质量分数)CGA组和98%(质量分数)CGA组,每组8只,分别给予磷酸缓冲盐溶液(phosphate buffer saline,PBS),50%CGA和98%CGA灌胃8周,每周检测体质量,每4周进行一次口服糖耐量试验(oral glucose tolerance test,OGTT),实验期末检测空腹胰岛素及胰岛素释放,计算稳态模型胰岛素抵抗指数(homeostasis model assessment insulin resistance,HOMA-IR)和内脏脂肪百分比,苏木精和伊红(hematoxylin and eosin,HE)染色检测胰腺组织病理变化.结果: CGA干预前,与CON组相比,HFD组OGTT第120分时(OGTT-120min)血糖值(P<0.05)和葡萄糖曲线下面积(area under curve-glucose,AUC-G)(P<0.05)均显著升高;干预4周后葡萄糖峰值时间延迟(P<0.05);干预8周 HOMA-IR指数显著升高且OGTT-0min,OGTT-30min,OGTT-60min,OGTT-120min胰岛素水平和胰岛素曲线下面积(area under curve-insulin,AUC-I)显著升高(P<0.05), 胰腺胰岛显著增生(P<0.05).与HFD组相比,干预4周末,50%CGA和98%CGA组大鼠糖耐量及其葡萄糖峰值时间均无显著变化;干预8周后,50%CGA组大鼠OGTT-60min,OGTT-120min血糖值,HOMA-IR指数,OGTT-0min,OGTT-30min,OGTT-120min血清胰岛素水平显著降低(P<0.05);98%CGA组大鼠OGTT-60min,OGTT-120min血糖值,HOMA-IR指数,OGTT-0min,OGTT-120min血清胰岛素水平显著降低(P<0.05);50%和98%CGA组大鼠的葡萄糖峰值时间均显著前移(P<0.05), 胰岛异常增生改善(P<0.05).结论: OGTT葡萄糖峰值时间延迟是DIO大鼠糖耐量异常表现之一,50%和98%CGA均可改善DIO大鼠的糖耐量和葡萄糖峰值时间延迟.
中图分类号:
- R587.1
| [1] | Wang L, Gao P, Zhang M , et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013[J]. JAMA, 2017,317(24):2515-2523. |
| [2] | Li G, Zhang P, Wang J , et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the da qing diabetes prevention study: a 23-year follow-up study[J]. Lancet Diabetes Endocrinol, 2014,2(6):474-480. |
| [3] | Unwin N, Shaw J, Zimmet P , et al. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention[J]. Diabet Med, 2002,19(9):708-723. |
| [4] | Hulman A, Witte DR, Vistisen D , et al. Pathophysiological characteristics underlying different glucose response curves: a latent class trajectory analysis from the prospective EGIR-RISC study[J]. Diabetes Care, 2018,41(8):1740-1748. |
| [5] | Tura A, Morbiducci U, Sbrignadello S , et al. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance[J]. Am J Physiol Regul Integr Comp Physiol, 2011,300(4):R941-R948. |
| [6] | Abdul-Ghani MA, Williams K, Defronzo R , et al. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose[J]. Diabetes Care, 2006,29(7):1613-1618. |
| [7] | Hayashi T, Boyko EJ, Sato KK , et al. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans[J]. Diabetes Care, 2013,36(5):1229-1235. |
| [8] | Kramer CK, Vuksan V, Choi H , et al. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: reproducibility and implications for glucose homeostasis in individuals with and without diabetes[J]. Diabetes Res Clin Pract, 2014,105(1):88-95. |
| [9] | Wang X, Zhao X, Zhou R , et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients[J]. J Diabetes Investig, 2018,9(6):1288-1295. |
| [10] | Kramer CK, Ye C, Hanley AJ , et al. Delayed timing of post-challenge peak blood glucose predicts declining beta cell function and worsening glucose tolerance over time: insight from the first year postpartum[J]. Diabetologia, 2015,58(6):1354-1362. |
| [11] | Kanauchi M, Kimura K, Kanauchi K , et al. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals[J]. Int J Clin Pract, 2005,59(4):427-432. |
| [12] | Chung ST, Ha J, Onuzuruike AU , et al. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk[J]. Clin Endocrinol (Oxf), 2017,87(5):484-491. |
| [13] | Li G, Zhang P, Wang J , et al. The long-term effect of lifestyle interventions to prevent diabetes in the China da qing diabetes prevention study: a 20-year follow-up study[J]. Lancet, 2008,371(9626):1783-1789. |
| [14] | Zuniga LY , Aceves-de LMM, Gonzalez-Ortiz M, et al. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance[J]. J Med Food, 2018,21(5):469-473. |
| [15] | Ma Y, Gao M, Liu D . Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice[J]. Pharm Res, 2015,32(4):1200-1209. |
| [16] | Jin S, Chang C, Zhang L , et al. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice[J]. PLoS One, 2015,10(4):e120842. |
| [17] | Panchal SK, Poudyal H, Waanders J , et al. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats[J]. J Nutr, 2012,142(4):690-697. |
| [18] | Hariri N, Thibault L . High-fat diet-induced obesity in animal models[J]. Nutr Res Rev, 2010,23(2):270-299. |
| [19] | Le Floch JP, Escuyer P, Baudin E , et al. Blood glucose area under the curve. Methodological aspects[J]. Diabetes Care, 1990,13(2):172-175. |
| [20] | Tschritter O, Fritsche A, Shirkavand F , et al. Assessing the shape of the glucose curve during an oral glucose tolerance test[J]. Diabetes Care, 2003,26(4):1026-1033. |
| [21] | Wong SK, Chin KY, Suhaimi FH , et al. The effects of a modified high-carbohydrate high-fat diet on metabolic syndrome parameters in male rats[J]. Exp Clin Endocrinol Diabetes, 2018,126(4):205-212. |
| [22] | Joung KH, Ju SH, Kim JM , et al. Clinical implications of using post-challenge plasma glucose levels for early diagnosis of type 2 diabetes mellitus in older individuals[J]. Diabetes Metab J, 2018,42(2):147-154. |
| [1] | 陈敬,单蕊,肖伍才,张晓蕊,刘峥. 青春期和成年早期自制力与抑郁症状和超重肥胖共病风险的关联:基于全国调查的十年前瞻性队列研究[J]. 北京大学学报(医学版), 2024, 56(3): 397-402. |
| [2] | 吴一凡,玉应香,谢岚,张志达,常翠青. 不同体重指数青年男性的静息能量消耗特点及预测方程评价[J]. 北京大学学报(医学版), 2024, 56(2): 247-252. |
| [3] | 陈楚云,孙蓬飞,赵静,贾佳,范芳芳,王春燕,李建平,姜一梦,霍勇,张岩. 北京社区人群促红细胞生成素相关因素及其与10年心血管疾病风险的关系[J]. 北京大学学报(医学版), 2023, 55(6): 1068-1073. |
| [4] | 党佳佳,蔡珊,钟盼亮,王雅琪,刘云飞,师嫡,陈子玥,张依航,胡佩瑾,李晶,马军,宋逸. 室外夜间人工光暴露与中国9~18岁儿童青少年超重肥胖的关联[J]. 北京大学学报(医学版), 2023, 55(3): 421-428. |
| [5] | 陈敬,肖伍才,单蕊,宋洁云,刘峥. DRD2基因rs2587552多态性对儿童肥胖干预效果的影响:一项前瞻性、平行对照试验[J]. 北京大学学报(医学版), 2023, 55(3): 436-441. |
| [6] | 马涛,李艳辉,陈曼曼,马莹,高迪,陈力,马奇,张奕,刘婕妤,王鑫鑫,董彦会,马军. 青春期启动提前与儿童肥胖类型的关联研究: 基于横断面调查和队列调查[J]. 北京大学学报(医学版), 2022, 54(5): 961-970. |
| [7] | 朱忆颖,闵赛南,俞光岩. 局部注射环孢素A对非肥胖糖尿病小鼠下颌下腺分泌功能及炎症的影响[J]. 北京大学学报(医学版), 2021, 53(4): 750-757. |
| [8] | 那晓娜,朱珠,陈阳阳,王东平,王浩杰,宋阳,马晓川,王培玉,刘爱萍. 身体活动、静坐行为的时间分布与肥胖的关系[J]. 北京大学学报(医学版), 2020, 52(3): 486-491. |
| [9] | 张晓圆,郭成成,玉应香,谢岚,常翠青. 高脂饲料诱导肥胖胰岛素抵抗大鼠模型的建立[J]. 北京大学学报(医学版), 2020, 52(3): 557-563. |
| [10] | 董彦会,宋逸,董彬,邹志勇,王政和,杨招庚,王西婕,李艳辉,马军. 2014年中国7~18岁学生血压状况与营养状况的关联分析——基于中国儿童青少年血压评价标准[J]. 北京大学学报(医学版), 2018, 50(3): 422-428. |
| [11] | 吴宇佳,迟晓培,陈峰,邓旭亮. 肥胖者唾液微生物宏基因组学特点[J]. 北京大学学报(医学版), 2018, 50(1): 5-12. |
| [12] | 杜依青,刘慧鑫,刘春雷,顿耀军,李清,于路平,刘士军,陈黎黎,王晓峰,徐涛. 代谢相关因素与肾细胞癌分级、分期的相关研究[J]. 北京大学学报(医学版), 2016, 48(4): 612-617. |
| [13] | 程兰,李钦,宋逸,马军,王海俊. 中国9~11岁小学生体育锻炼、静态行为和#br# 超重与肥胖的关系[J]. 北京大学学报(医学版), 2016, 48(3): 436-441. |
| [14] | 付连国, 王海俊, 阳益德, 李晓卉, 王烁, 孟祥坤, 王政和, 马军. 儿童青少年对肥胖危险因素知晓的现状分析[J]. 北京大学学报(医学版), 2015, 47(3): 410-413. |
| [15] | 温萌萌, 朱广荣, 王海雪. 中国11~18岁汉族男生肥胖与首次遗精年龄的相关性分析[J]. 北京大学学报(医学版), 2015, 47(3): 406-409. |
|
||