北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (4): 770-775. doi: 10.19723/j.issn.1671-167X.2021.04.025
飞秒激光表面处理对氧化锆表面特征及弯曲强度的影响
李文锦1,丁茜1,原福松2,孙丰博3,郑剑桥2,鲍蕊4,张磊1,Δ( )
)
- 1.北京大学口腔医学院·口腔医院,修复科 国家口腔医学中心 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室,北京 100081
2.北京大学口腔医学院·口腔医院,口腔医学数字化研究中心 口腔数字医学北京市重点实验室 国家卫生健康委口腔医学计算机应用工程技术研究中心,北京 100081
3.清华大学材料学院,北京 100084
4.北京航空航天大学航空科学与工程学院,北京 100191
Effects of femtosecond laser treatment on surface characteristics and flexural strength of zirconia
LI Wen-jin1,DING Qian1,YUAN Fu-song2,Sun Feng-bo3,ZHENG Jian-qiao2,BAO Rui4,Zhang Lei1,Δ( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
2. Center for Digital Dentistry, Peking University School and Hospital of Stomatology & Beijing Key Laboratory of Digital Stomatology & Research Center of Engineering and Technology for Computerized Dentistry Ministry of Health, Beijing 100081, China
3. School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
4. School of Aeronautic Science and Engineering, Beihang University, Beijing 100191, China
摘要:
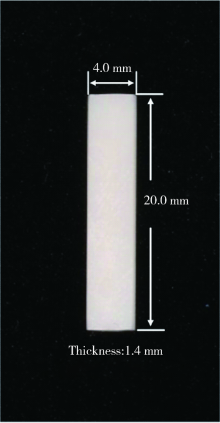
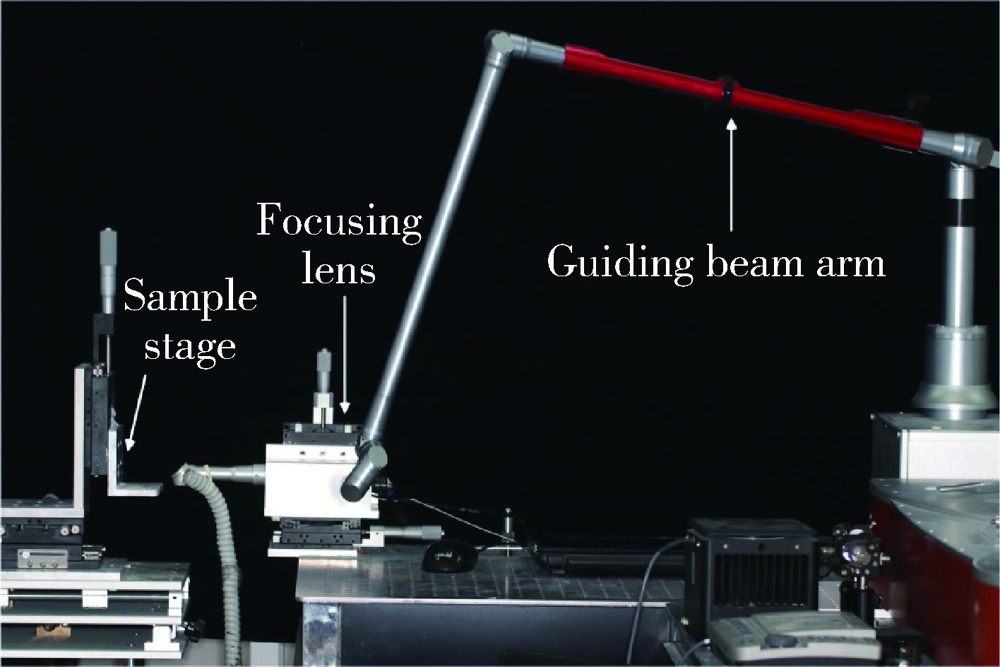
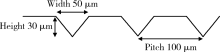
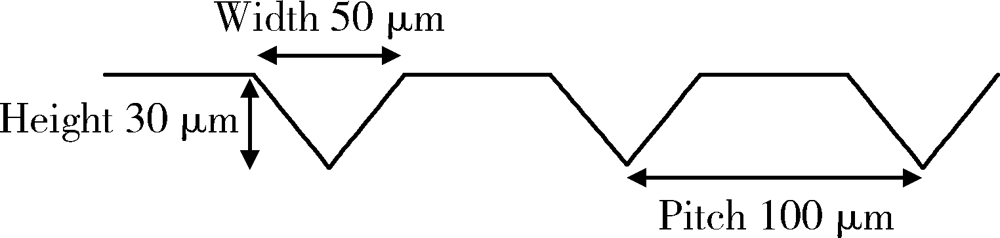
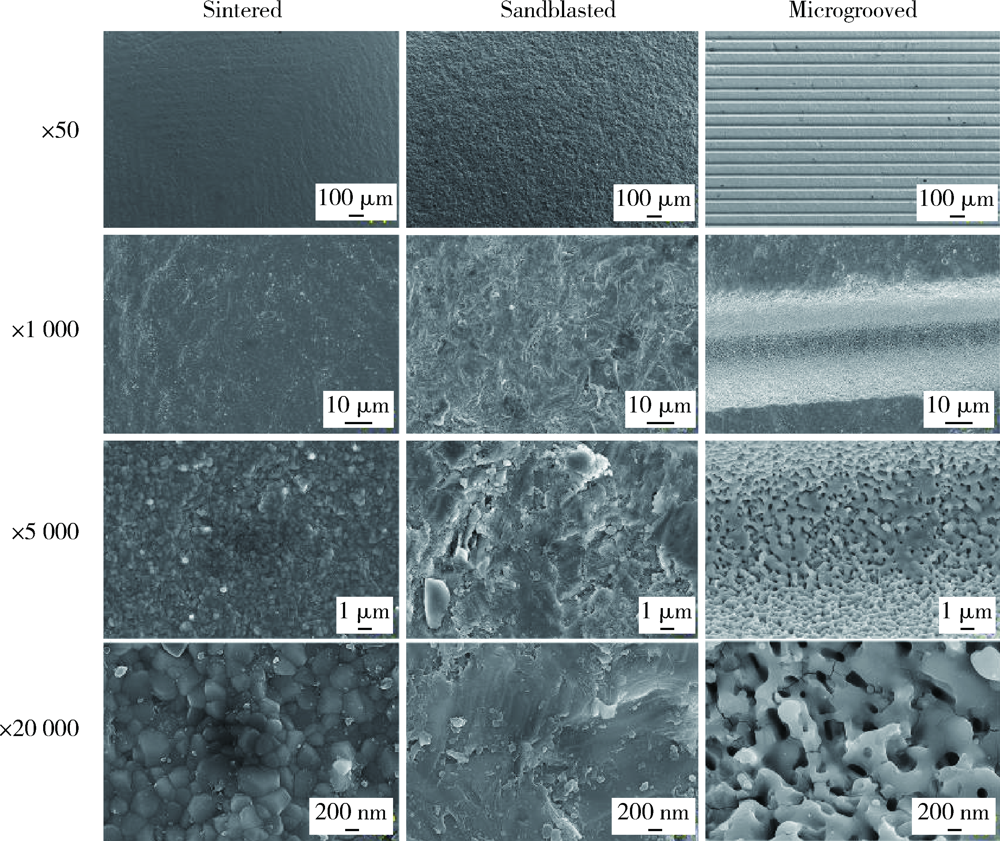
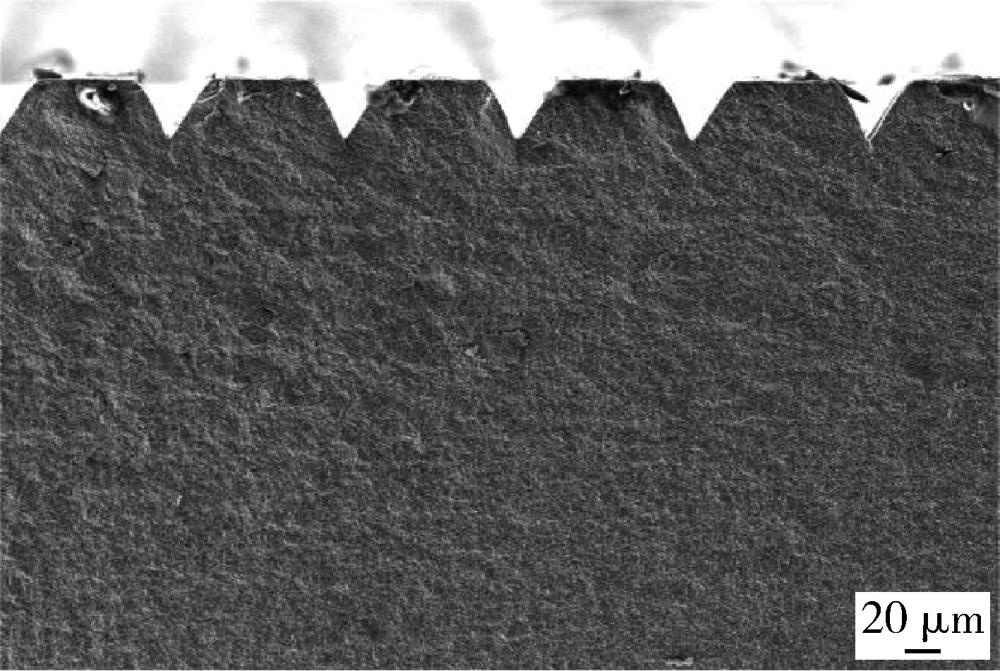
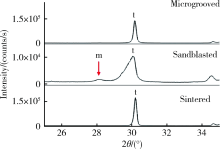
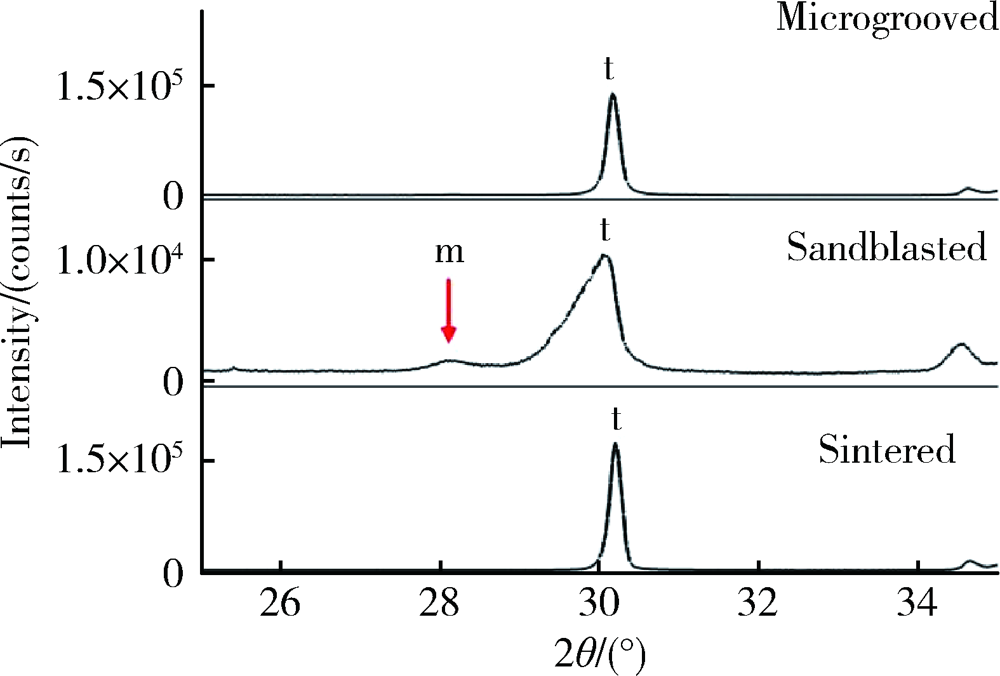
目的: 研究飞秒激光表面处理后获得的微沟槽结构对氧化锆表面显微形貌、晶相组成及弯曲强度的影响,为氧化锆种植体表面微观结构优化提供参考。方法: 根据不同表面处理方法,将57个计算机辅助设计/计算机辅助制造(computer aided design/computer aided manufacture,CAD/CAM)的长方体氧化锆标准试件(20.0 mm×4.0 mm×1.4 mm)分为3组(每组19个):(1)终烧结组,终烧结后无处理,作为对照;(2)喷砂组,终烧结后用110 μm氧化铝(Al2O3)喷砂;(3)微沟槽组,用飞秒激光加工宽50 μm、深30 μm、间距100 μm的微沟槽。通过扫描电镜和3D激光形貌测量显微镜观察表面显微形貌,计算各组表面粗糙度和微沟槽组的沟槽尺寸,采用X射线衍射仪进行晶相分析,进行三点弯曲试验,通过Weibull分布分析其强度特征。结果: 扫描电镜显示终烧结组表面较为平整,晶粒结构清晰,喷砂组表面凹凸不平,出现边缘锐利的凹坑,形状不规则,微沟槽组表面微沟槽排列规则,未见明显缺陷,沟槽内壁形成纳米级颗粒状显微结构。微沟槽组粗糙度Ra值(9.42±0.28) μm显著高于喷砂组(1.04±0.03) μm和终烧结组(0.60±0.04) μm,喷砂组与终烧结组之间差异亦有统计学意义(P<0.001)。飞秒激光加工的微沟槽尺寸精确,宽度(49.75±1.24) μm,深度(30.85±1.02) μm,间距(100.58±1.94) μm;晶相分析结果显示,喷砂组的单斜相体积百分数(18.17%)较终烧结组(1.55%)明显增加,微沟槽组(2.21%)与终烧结组相近;喷砂组的弯曲强度(986.22±163.25) MPa与终烧结组(946.46±134.15) MPa相比差异无统计学意义(P=0.847),而微沟槽组弯曲强度(547.92±30.89) MPa较其余两组显著下降(P<0.001);终烧结组、喷砂组、微沟槽组的Weibull模数m分别为7.89、6.98、23.46。结论: 飞秒激光处理可在氧化锆表面形成具有微纳结构的微沟槽,会显著降低氧化锆的弯曲强度。
中图分类号:
- R783.4
| [1] |
Scarano A, di Carlo F, Quaranta M, et al. Bone response to zirconia ceramic implants: an experimental study in rabbits [J]. J Oral Implantol, 2003, 29(1):8-12.
doi: 10.1563/1548-1336(2003)029<0008:BRTZCI>2.3.CO;2 |
| [2] |
Spies BC, Nold J, Vach K, et al. Two-piece zirconia oral implants withstand masticatory loads: An investigation in the artificial mouth [J]. J Mech Behav Biomed Mater, 2016, 53:1-10.
doi: 10.1016/j.jmbbm.2015.07.005 |
| [3] |
Scarano A, Piattelli M, Caputi S, et al. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study [J]. J Periodontol, 2004, 75(2):292-296.
doi: 10.1902/jop.2004.75.2.292 |
| [4] |
Borgonovo AE, Censi R, Vavassori V, et al. Zirconia implants in esthetic areas: 4-year follow-up evaluation study [J]. Int J Dent, 2015, 2015:415029.
doi: 10.1155/2015/415029 pmid: 26124836 |
| [5] | Fischer J, Schott A, Martin S. Surface micro-structuring of zirconia dental implants [J]. Clin Oral Implants Res, 2016, 27(2):162-166. |
| [6] |
Pardun K, Treccani L, Volkmann E, et al. Magnesium-containing mixed coatings on zirconia for dental implants: mechanical characterization and in vitro behavior [J]. J Biomater Appl, 2015, 30(1):104-118.
doi: 10.1177/0885328215572428 |
| [7] |
Delgado-Ruíz RA, Calvo-Guirado JL, Moreno P, et al. Femto-second laser microstructuring of zirconia dental implants [J]. J Biomed Mater Res B, 2011, 96(1):91-100.
doi: 10.1002/jbm.b.31743 pmid: 21061361 |
| [8] |
Delgado-Ruíz RA, Gomez MG, Aguilar-Salvatierra A, et al. Human fetal osteoblast behavior on zirconia dental implants and zirconia disks with microstructured surfaces. An experimental in vitro study [J]. Clin Oral Implants Res, 2016, 27(11):e144-e153.
doi: 10.1111/clr.12585 |
| [9] |
Delgado-Ruiz RA, Abboud M, Romanos G, et al. Peri-implant bone organization surrounding zirconia-microgrooved surfaces circularly polarized light and confocal laser scanning microscopy study [J]. Clin Oral Implants Res, 2015, 26(11):1328-1337.
doi: 10.1111/clr.2015.26.issue-11 |
| [10] | Pilkey WD, Pilkey DF. Peterson’s stress concentration factors[M]. 3rd ed. New Jersey: John Wiley & Sons, 2008: 3-9. |
| [11] |
Garvie RC, Nicholson PS. Phase analysis in zirconia systems [J]. J Am Ceram Soc, 1972, 55(6):303-305.
doi: 10.1111/jace.1972.55.issue-6 |
| [12] | Toraya H, Yoshimura M, Somiya S. Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction [J]. J Am Ceram Soc, 1984, 67(6):119-121. |
| [13] | British Standards Institution. ISO 6872: 2015, Dentistry-ceramic materials[S]. London: BSI Standards Publication, 2015. |
| [14] |
Quinn JB, Quinn GD. A practical and systematic review of Weibull statistics for reporting strengths of dental materials [J]. Dent Mater, 2009, 26(2):135-147.
doi: 10.1016/j.dental.2009.09.006 |
| [15] |
Kurella A, Dahotre NB. Review paper: surface modification for bioimplants: the role of laser surface engineering [J]. J Biomater Appl, 2005, 20(1):5-50.
pmid: 15972362 |
| [16] | 孙玉春, Vorobyev A, 刘晶, 等. 飞秒激光切削牙齿硬组织表面粗糙度和显微形貌观察 [J]. 中华口腔医学杂志, 2012, 47(8):486-489. |
| [17] | Roitero E, Lasserre F, Anglada M, et al. A parametric study of laser interference surface patterning of dental zirconia: Effects of laser parameters on topography and surface quality [J]. Dent Mater, 2016, 33(1):e28-e38. |
| [18] |
Ricci JL, Grew JC, Alexander H. Connective-tissue responses to defined biomaterial surfaces. Ⅰ. Growth of rat fibroblast and bone marrow cell colonies on microgrooved substrates [J]. J Biomed Mater Res A, 2008, 85A(2):313-325.
doi: 10.1002/(ISSN)1552-4965 |
| [19] |
Khandaker M, Riahinezhad S, Williams WR, et al. Microgroove and collagen-poly(ε-caprolactone) nanofiber mesh coating improves the mechanical stability and osseointegration of titanium implants [J]. Nanomaterials, 2017, 7(6):145.
doi: 10.3390/nano7060145 |
| [20] |
Souza JCM, Sordi MB, Kanazawa M, et al. Nano-scale modification of titanium implant surfaces to enhance osseointegration [J]. Acta Biomater, 2019, 94:112-131.
doi: 10.1016/j.actbio.2019.05.045 |
| [21] |
Ding Q, Zhang L, Bao R, et al. Effects of different surface treatments on the cyclic fatigue strength of one-piece CAD/CAM zirconia implants [J]. J Mech Behav Biomed Mater, 2018, 84:249-257.
doi: 10.1016/j.jmbbm.2018.05.002 |
| [22] |
Frandsen CJ, Noh K, Brammer KS, et al. Hybrid micro/nano-topography of a TiO2 nanotube-coated commercial zirconia femoral knee implant promotes bone cell adhesion in vitro [J]. Mater Sci Eng C, 2013, 33(5):2752-2756.
doi: 10.1016/j.msec.2013.02.045 |
| [23] |
Weiss P, Garber B. Shape and movement of mesenchyme cells as functions of the physical structure of the medium. Contributions to a quantitative morphology [J]. PNAS, 1952, 38(3):264-280.
pmid: 16589090 |
| [24] |
Lee M, Oh N, Lee S, et al. Factors influencing osteoblast maturation on microgrooved titanium substrata [J]. Biomaterials, 2010, 31(14):3804-3815.
doi: 10.1016/j.biomaterials.2010.01.117 |
| [25] |
Carvalho A, Cangueiro L, Oliveira V, et al. Femtosecond laser microstructured Alumina toughened Zirconia: A new strategy to improve osteogenic differentiation of hMSCs [J]. Appl Surf Sci, 2018, 435:1237-1245.
doi: 10.1016/j.apsusc.2017.11.206 |
| [26] |
de Luca AC, Zink M, Weidt A, et al. Effect of microgrooved surface topography on osteoblast maturation and protein adsorption [J]. J Biomed Mater Res A, 2015, 103(8):2689-2700.
doi: 10.1002/jbm.a.v103.8 |
| [27] |
Guilardi LF, Soares P, Werner A, et al. Fatigue performance of distinct CAD/CAM dental ceramics [J]. J Mech Behav Biomed Mater, 2019, 103:103540.
doi: 10.1016/j.jmbbm.2019.103540 |
| [28] |
Iseri U, Ozkurt Z, Yalniz A, et al. Comparison of different grinding procedures on the flexural strength of zirconia [J]. J Prosthet Dent, 2012, 107(5):309-315.
doi: 10.1016/S0022-3913(12)60081-X |
| [29] |
Aboushelib MN, Wang H, Kleverlaan CJ, et al. Fatigue behavior of zirconia under different loading conditions [J]. Dent Mater, 2016, 32(7):915-920.
doi: 10.1016/j.dental.2016.03.012 pmid: 27063462 |
| [30] |
Piconi C, Maccauro G. Zirconia as a ceramic biomaterial [J]. Biomaterials, 1999, 20(1):1-25.
pmid: 9916767 |
| [31] | 龚旭, 赵信义, 张春宝, 等. 喷砂对口腔氧化锆陶瓷抗弯强度和亚临界裂纹扩展的影响 [J]. 中华口腔医学杂志, 2017, 52(7):439-442. |
| [32] |
Kosmac T, Oblak C, Jevnikar P, et al. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic [J]. Dent Mater, 1999, 15(6):426-433.
pmid: 10863444 |
| [33] |
Aivazi M, Hossein FM, Nejatidanesh F, et al. The evaluation of prepared microgroove pattern by femtosecond laser on alumina-zirconia nano-composite for endosseous dental implant application [J]. Lasers Med Sci, 2016, 31(9):1837-1843.
doi: 10.1007/s10103-016-2059-8 |
| [34] |
Chintapalli RK, Marro FG, Jimenez-Pique E, et al. Phase transformation and subsurface damage in 3Y-TZP after sandblasting [J]. Dent Mater, 2013, 29(5):566-572.
doi: 10.1016/j.dental.2013.03.005 pmid: 23537568 |
| [35] |
Karakoca S, Yilmaz H. Influence of surface treatments on surface roughness, phase transformation, and biaxial flexural strength of Y-TZP ceramics [J]. J Biomed Mater Res B, 2009, 91(2):930-937.
doi: 10.1002/jbm.b.31477 pmid: 19637376 |
| [36] | Taylor D. The theory of critical distances: a new perspective in fracture mechanics[M]. London:Elsevier, 2007: 8-10. |
| [1] | 丁茜,李文锦,孙丰博,谷景华,林元华,张磊. 表面处理对氧化钇和氧化镁稳定的氧化锆种植体晶相及断裂强度的影响[J]. 北京大学学报(医学版), 2023, 55(4): 721-728. |
| [2] | 李伟伟,陈虎,王勇,孙玉春. 氧化锆陶瓷表面硅锂喷涂层的摩擦磨损性能[J]. 北京大学学报(医学版), 2023, 55(1): 94-100. |
| [3] | 王铮,丁茜,高远,马全诠,张磊,葛兮源,孙玉春,谢秋菲. 氧化锆多孔表面显微形貌对成骨细胞增殖及分化的影响[J]. 北京大学学报(医学版), 2022, 54(1): 31-39. |
| [4] | 杨欣,李榕,叶红强,陈虎,王勇,周永胜,孙玉春. 不同刃状边缘补偿角度的两种氧化锆全瓷冠断裂强度的评价[J]. 北京大学学报(医学版), 2021, 53(2): 402-405. |
| [5] | 郑苗,詹凌璐,刘志强,李和平,谭建国. 不同等离子体处理氧化锆对人牙龈成纤维细胞黏附能力的影响[J]. 北京大学学报(医学版), 2019, 51(2): 315-320. |
| [6] | 周团锋,王新知. 计算机辅助设计与制作的一体化氧化锆全瓷桩核5年临床观察[J]. 北京大学学报(医学版), 2018, 50(4): 680-684. |
| [7] | 崔新悦,佟岱,王新知,沈志坚. 对比切削与胶态成型工艺制作的氧化锆陶瓷的透光性与遮色效果[J]. 北京大学学报(医学版), 2018, 50(1): 85-90. |
| [8] | 廖宇,刘晓强,陈立,周建锋,谭建国. 不同表面处理方法对氧化锆与树脂水门汀粘接强度的影响[J]. 北京大学学报(医学版), 2018, 50(1): 53-57. |
| [9] | 焦洋, 王继德, 邓久鹏. 不同表面处理对氧化锆晶相结构及性能的影响[J]. 北京大学学报(医学版), 2018, 50(1): 49-52. |
| [10] | 周团锋, 张相皞, 王新知. 一体化计算机辅助设计与制作氧化锆桩核的三维有限元分析[J]. 北京大学学报(医学版), 2015, 47(1): 78-84. |
| [11] | 吕品, 姜婷. 热酸蚀处理对氧化锆表面剪切粘接强度的影响[J]. 北京大学学报(医学版), 2014, 46(2): 302-305. |
| [12] | 周团锋,张相皞,王新知. 计算机辅助设计与制作的一体化氧化锆全瓷桩核抗折力的体外对比实验[J]. 北京大学学报(医学版), 2014, 46(1): 81-85. |
| [13] | 刘亦洪,王勇,张庆辉,高原,冯海兰. 基底冠设计对氧化锆全瓷冠抗力的影响[J]. 北京大学学报(医学版), 2014, 46(1): 71-75. |
| [14] | 孙玉春, Anatoliy Vorobyev, 李虹, Chunlei Guo, 王勇. 特定参数飞秒激光制备离体牙釉质窝洞对髓腔温度的影响[J]. 北京大学学报(医学版), 2013, 45(2): 286-. |
| [15] | 周团锋, 王新知Δ. 三种不同直径一体化计算机辅助设计与制作氧化锆全瓷桩核修复的有限元法分析[J]. 北京大学学报(医学版), 2012, 44(1): 93-97. |
|
||