北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (5): 934-938. doi: 10.19723/j.issn.1671-167X.2023.05.024
比较Epsilometer试验法和琼脂稀释法检测幽门螺杆菌对甲硝唑的敏感性
- 北京大学第三医院消化科, 北京 100191
Comparison of Epsilometer test and agar dilution method in detecting the sensitivity of Helicobacter pylori to metronidazole
Xue-li TIAN,Zhi-qiang SONG*( ),Bao-jun SUO,Li-ya ZHOU,Cai-ling LI,Yu-xin ZHANG
),Bao-jun SUO,Li-ya ZHOU,Cai-ling LI,Yu-xin ZHANG
- Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China
摘要:
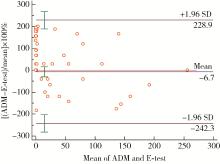
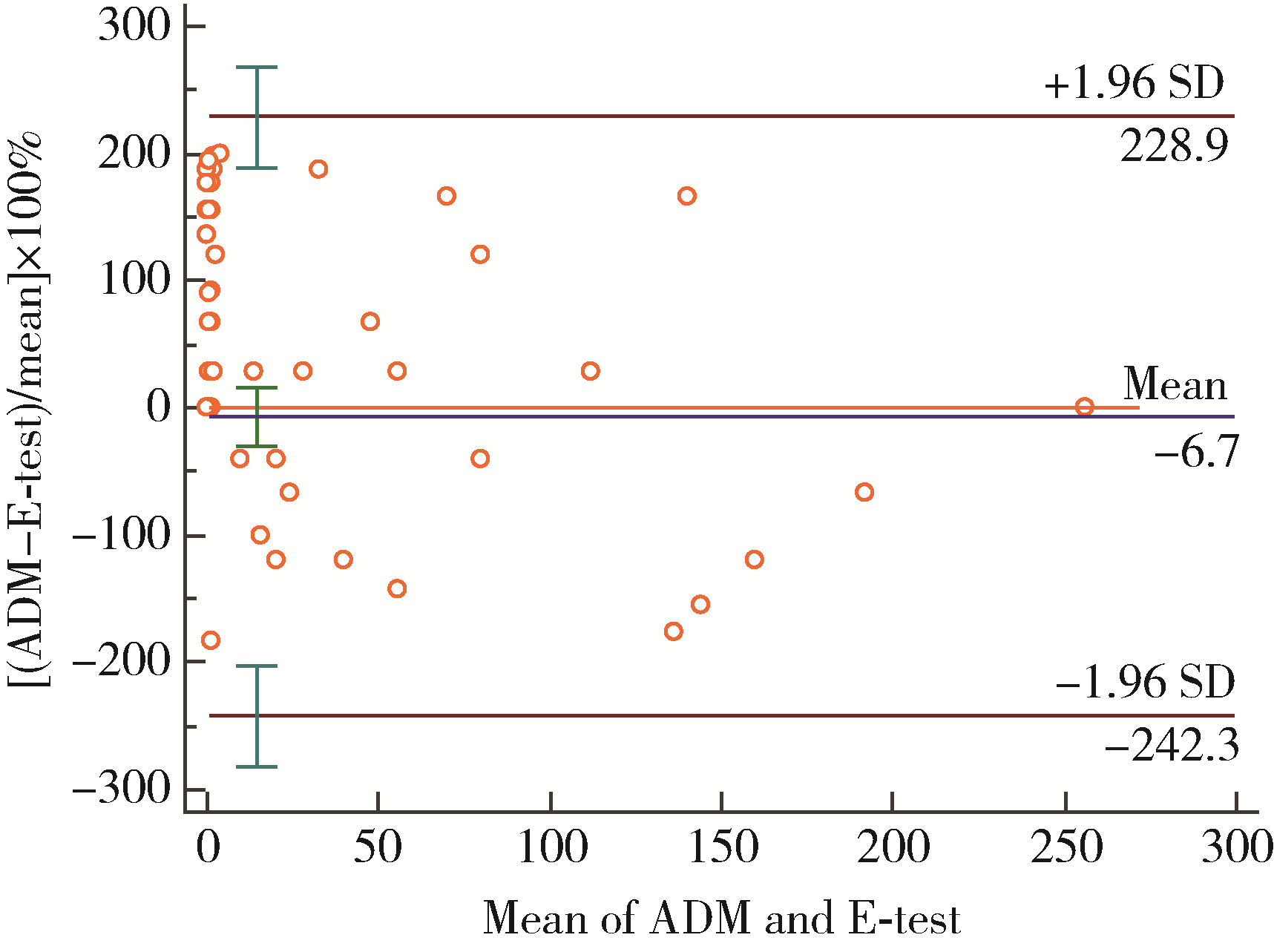
目的: 以琼脂稀释法为金标准, 评价Epsilometer试验(Epsilometer test, E-test)法检测幽门螺杆菌(Helicobacter pylori, H. pylori)对甲硝唑敏感性的一致性。方法: 纳入2018年8月至2020年7月因消化不良症状就诊于北京大学第三医院行胃镜检查的H. pylori感染初治患者, 取胃黏膜组织活检行H. pylori培养, 分别采用E-test法和琼脂稀释法检测H. pylori对甲硝唑的敏感性, 比较两种方法检测结果的一致性和相关性。结果: 成功培养105株H. pylori, 将最小抑菌浓度≥ 8 mg/L定义为耐药。琼脂稀释法检测甲硝唑耐药菌株68株, 耐药率64.8%, E-test法检测耐药菌株66株, 耐药率62.9%, 其中, 琼脂稀释法和E-test法检测均为耐药的菌株66株, 均为敏感的菌株37株, 两种方法的一致率为98.1%。2例菌株被琼脂稀释法评价为耐药, 而E-test法评价为敏感, 非常严重错误率为1.9%。没有菌株被琼脂稀释法评价为敏感, 而E-test法评价为耐药(严重错误率为0%)。以琼脂稀释法为金标准, E-test法检测甲硝唑耐药的灵敏度为97.1%(95%CI: 0.888~0.995), 特异度为100%(95%CI: 0.883~1.000)。Cohen’s kappa系数为0.959 (95%CI: 0.902~1.016, P < 0.001), Spearmans相关性检测r=0.807(P < 0.001)。采用Bland-Altman法进行一致性评价, 结果提示较好, 未出现一致性区间外的测值。E-test法比琼脂稀释法的成本更低, 平均完成1例试验两者的成本分别为269.8元和356.6元。结论: E-test法检测H. pylori对甲硝唑的药敏试验与琼脂稀释法相比具有较强的一致性, E-test法省时、省力、价廉, 可以作为H. pylori药敏试验的优选检测方法。
中图分类号:
- R446.5
| 1 |
Sugano K , Tack J , Kuipers EJ , et al. Kyoto global consensus report on Helicobacter pylori gastritis[J]. Gut, 2015, 64 (9): 1353- 1367.
doi: 10.1136/gutjnl-2015-309252 |
| 2 |
Malfertheiner P , Megraud F , OMorain CA , et al. Management of Helicobacter pylori infection: the Maastricht V/Florence consensus report[J]. Gut, 2017, 66 (1): 6- 30.
doi: 10.1136/gutjnl-2016-312288 |
| 3 |
Hu Y , Zhu Y , Lu NH . Primary antibiotic resistance of Helicobac-ter pylori in China[J]. Dig Dis Sci, 2017, 62 (5): 1146- 1154.
doi: 10.1007/s10620-017-4536-8 |
| 4 | FitzGerald R, Smith SM. An overview of Helicobacter pylori infection [M]// Methods in Molecular Biology. New York: Humana Press, 2021: 1-14. |
| 5 |
Thung I , Aramin H , Vavinskaya V , et al. Review article: The global emergence of Helicobacter pylori antibiotic resistance[J]. Aliment Pharmacol Ther, 2016, 43 (4): 514- 533.
doi: 10.1111/apt.13497 |
| 6 |
Pan J , Shi Z , Lin D , et al. Is tailored therapy based on antibiotic susceptibility effective? A multicenter, open-label, randomized trial[J]. Front Med, 2020, 14 (1): 43- 50.
doi: 10.1007/s11684-019-0706-8 |
| 7 |
Alarcon T , Domingo D , Lopez-Brea M . Discrepancies between E-test and agar dilution methods for testing metronidazole susceptibi-lity of Helicobacter pylori[J]. J Clin Microbiol, 1998, 36 (4): 1165- 1166.
doi: 10.1128/JCM.36.4.1165-1166.1998 |
| 8 |
Hachem CY , Clarridge JE , Reddy R , et al. Antimicrobial susceptibility testing of Helicobacter pylori, comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole[J]. Diagn Microbiol Infect Dis, 1996, 24 (1): 37- 41.
doi: 10.1016/0732-8893(95)00252-9 |
| 9 |
Miftahussurur M , Fauzia KA , Nusi IA , et al. E-test versus agar dilution for antibiotic susceptibility testing of Helicobacter pylori: A comparison study[J]. BMC Res Notes, 2020, 13 (1): 22.
doi: 10.1186/s13104-019-4877-9 |
| 10 | CL SI . Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically[M]. 10th ed Wayne: Clinical and Laboratory Standards Institute, 2015: M07. |
| 11 |
Alarcón T , Urruzuno P , Martínez MJ , et al. Antimicrobial susceptibility of 6 antimicrobial agents in Helicobacter pylori clinical isolates by using EUCAST breakpoints compared with previously used breakpoints[J]. Enferm Infecc Microbiol Clin, 2017, 35 (5): 278- 282.
doi: 10.1016/j.eimc.2016.02.010 |
| 12 |
Hooi JKY , Lai WY , Ng WK , et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis[J]. Gastroenterology, 2017, 153 (2): 420- 429.
doi: 10.1053/j.gastro.2017.04.022 |
| 13 |
刘文忠, 谢勇, 陆红, 等. 第五次全国幽门螺杆菌感染处理共识报告[J]. 中华内科杂志, 2017, 56 (7): 532- 545.
doi: 10.3760/cma.j.issn.0578-1426.2017.07.014 |
| 14 |
Mégraud F , Lehn N , Lind T , et al. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: The MACH2 study[J]. Antimicrob Agents Chemother, 1999, 43 (11): 2747- 2752.
doi: 10.1128/AAC.43.11.2747 |
| 15 |
Glupczynski Y , Broutet N , Cantagrel A , et al. Comparison of the E-test and agar dilution method for antimicrobial suceptibility testing of Helicobacter pylori[J]. Eur J Clin Microbiol Infect Dis, 2002, 21 (7): 549- 552.
doi: 10.1007/s10096-002-0757-6 |
| 16 |
Osato MS , Reddy R , Reddy SG , et al. Comparison of the E-test and the NCCLS-approved agar dilution method to detect metro-nidazole and clarithromycin resistant Helicobacter pylori[J]. Int J Antimicrob Agents, 2001, 17 (1): 39- 44.
doi: 10.1016/S0924-8579(00)00320-4 |
| 17 |
El-Halfawy OM , Valvano MA . Antimicrobial heteroresistance: An emerging field in need of clarity[J]. Clin Microbiol Rev, 2015, 28 (1): 191- 207.
doi: 10.1128/CMR.00058-14 |
| 18 | Ogata SK , Gales AC , Kawakami E . Antimicrobial susceptibility testing for Helicobacter pylori isolates from Brazilian children and adolescents: Comparing agar dilution, E-test, and disk diffusion[J]. Braz J Microbiol, 2015, 45 (4): 1439- 1448. |
| 19 |
Best LM , Haldane DJ , Keelan M , et al. Multilaboratory comparison of proficiencies in susceptibility testing of Helicobacter pylori and correlation between agar dilution and E-test methods[J]. Antimicrob Agents Chemother, 2003, 47 (10): 3138- 3144.
doi: 10.1128/AAC.47.10.3138-3144.2003 |
| 20 |
Valdivieso-García A , Imgrund R , Deckert A , et al. Cost analysis and antimicrobial susceptibility testing comparing the E-test and the agar dilution method in Campylobacter jejuni and Campylobacter coli[J]. Diagn Microbiol Infect Dis, 2009, 65 (2): 168- 174.
doi: 10.1016/j.diagmicrobio.2009.07.008 |
| [1] | 管仁珍, 丁士刚, 石岩岩, 薛艳. 含铋剂四联疗法根除幽门螺杆菌的疗效:4 261例患者的真实世界研究[J]. 北京大学学报(医学版), 2024, 56(5): 942-945. |
| [2] | 侯卫华,宋书杰,石中月,金木兰. 幽门螺杆菌阴性早期胃癌的临床病理特征[J]. 北京大学学报(医学版), 2023, 55(2): 292-298. |
| [3] | 王子靖,李在玲. 有幽门螺杆菌感染家族史儿童胃部菌群的特点[J]. 北京大学学报(医学版), 2021, 53(6): 1115-1121. |
| [4] | 包振英, 林琴, 孟彦宏, 何淳,苏家增, 彭歆. 厌氧菌检测技术在口腔颌面部感染治疗中的应用[J]. 北京大学学报(医学版), 2016, 48(1): 76-79. |
| [5] | 张路, 袁重阳, 田福聪, 王晓燕, 高学军. 自酸蚀粘接剂系统对变形链球菌的抑制作用[J]. 北京大学学报(医学版), 2016, 48(1): 57-62. |
| [6] | 张志平, 郭昭庆, 孙垂国, 曾岩, 李危石, 齐强, 陈仲强. 胸、腰椎后路内固定术后深部手术切口感染的微生物学分析[J]. 北京大学学报(医学版), 2015, 47(2): 358-360. |
| [7] | 王澍, 施永康, 黄晓波, 马凯, 许清泉, 熊六林, 李建兴, 王晓峰. 上尿路结石合并感染的细菌培养及药物敏感性分析[J]. 北京大学学报(医学版), 2014, 46(5): 798-801. |
| [8] | 石岩岩, 丁士刚, 张婷, 鲁凤民 , 张静, 王晔. 幽门螺杆菌硫氧还蛋白-1基因克隆及其重组蛋白表达与活性测定[J]. 北京大学学报(医学版), 2014, 46(2): 190-194. |
|
||