北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (6): 975-981. doi: 10.19723/j.issn.1671-167X.2023.06.004
干扰素-α介导系统性红斑狼疮外周血CD56dimCD57+自然杀伤细胞功能的损伤
- 大连医科大学基础医学院免疫学教研室, 辽宁大连 116044
Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss
Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI*( )
)
- Department of Immunology, College of Basic Medical Science, Dalian Medical University, Dalian 116044, Liaoning, China
摘要:
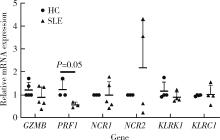
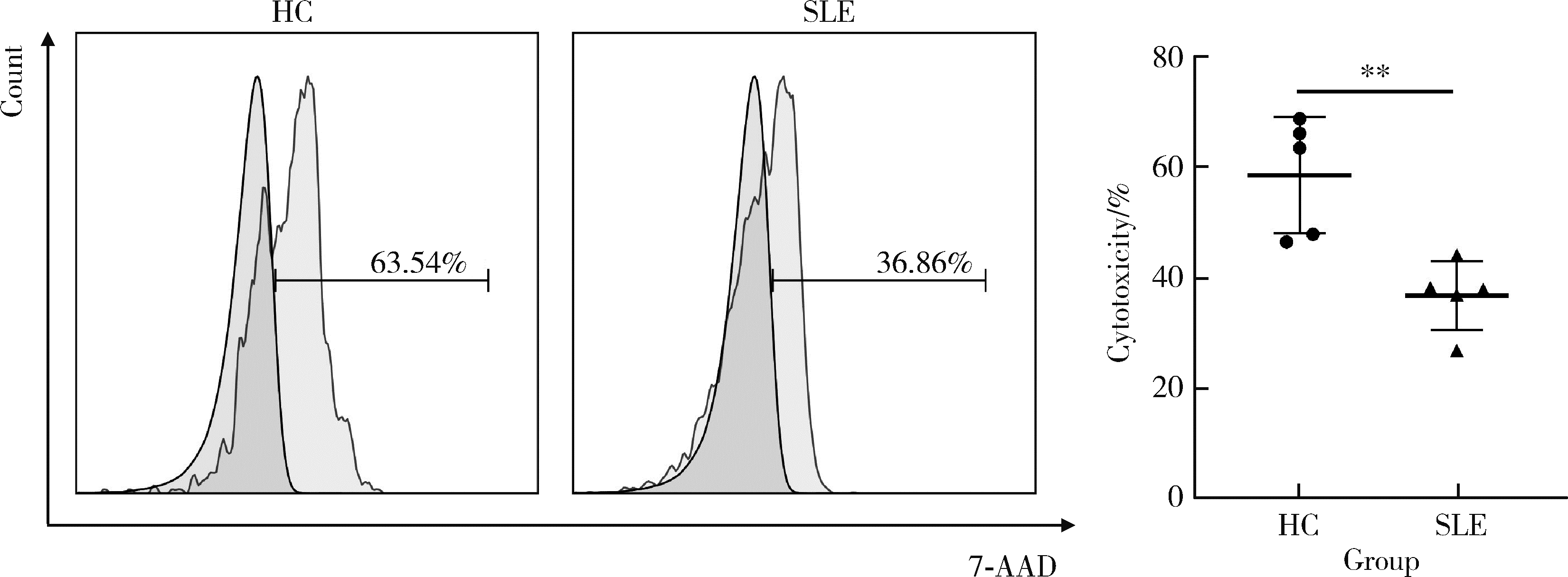
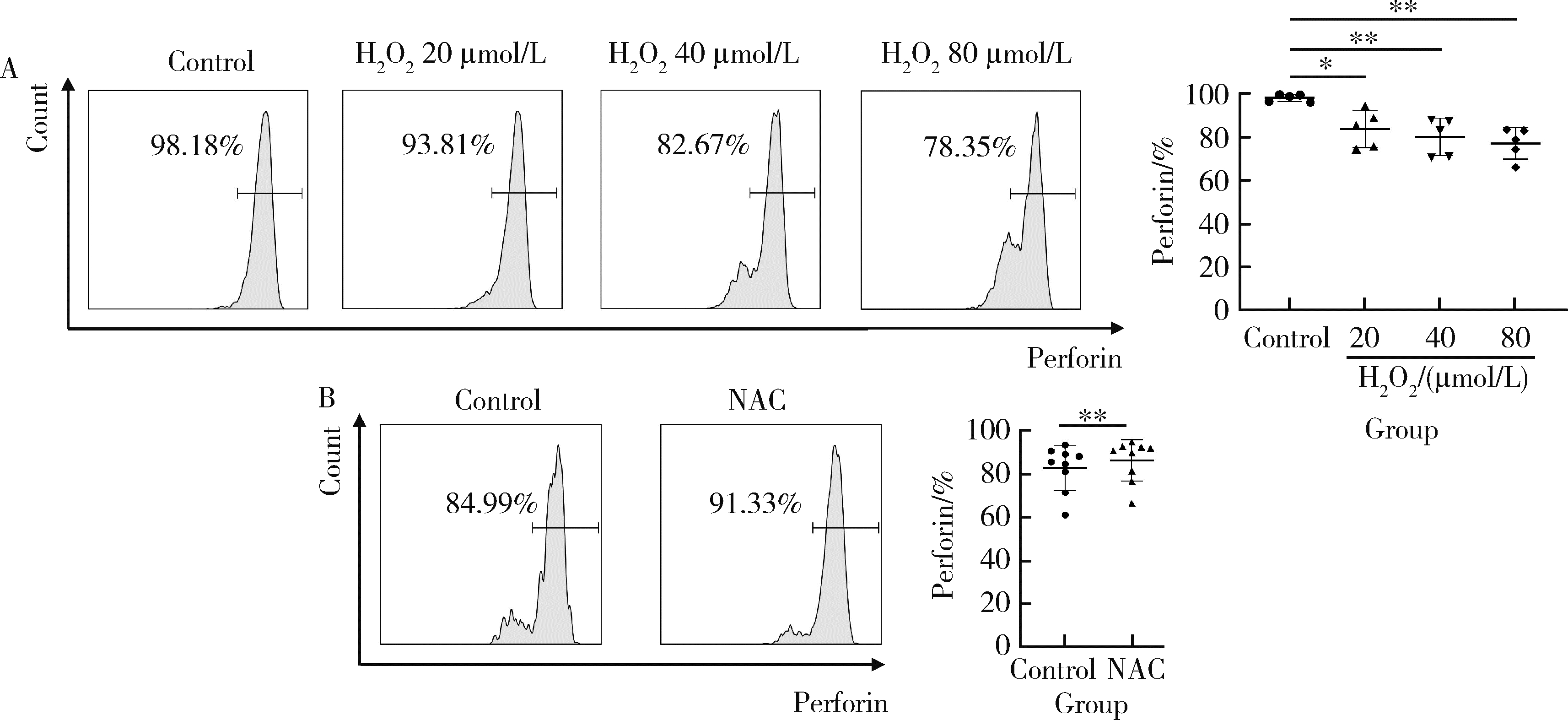
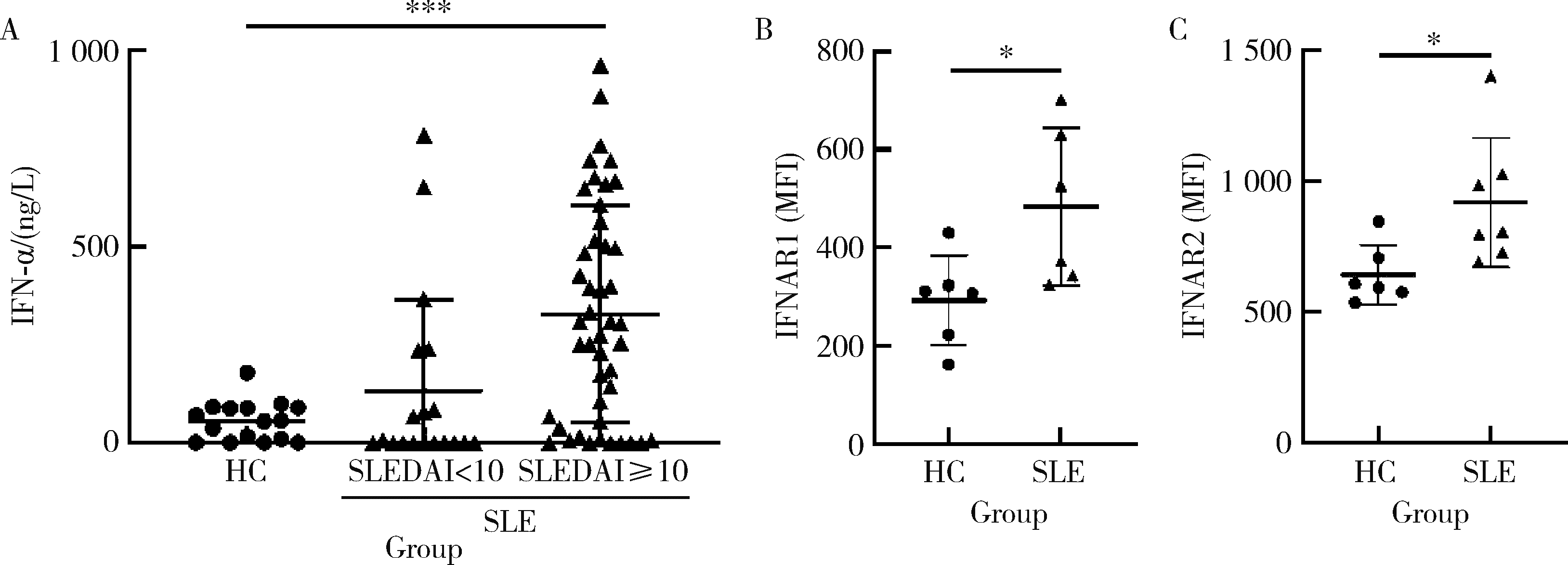
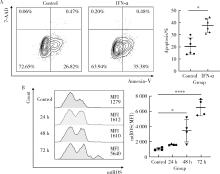
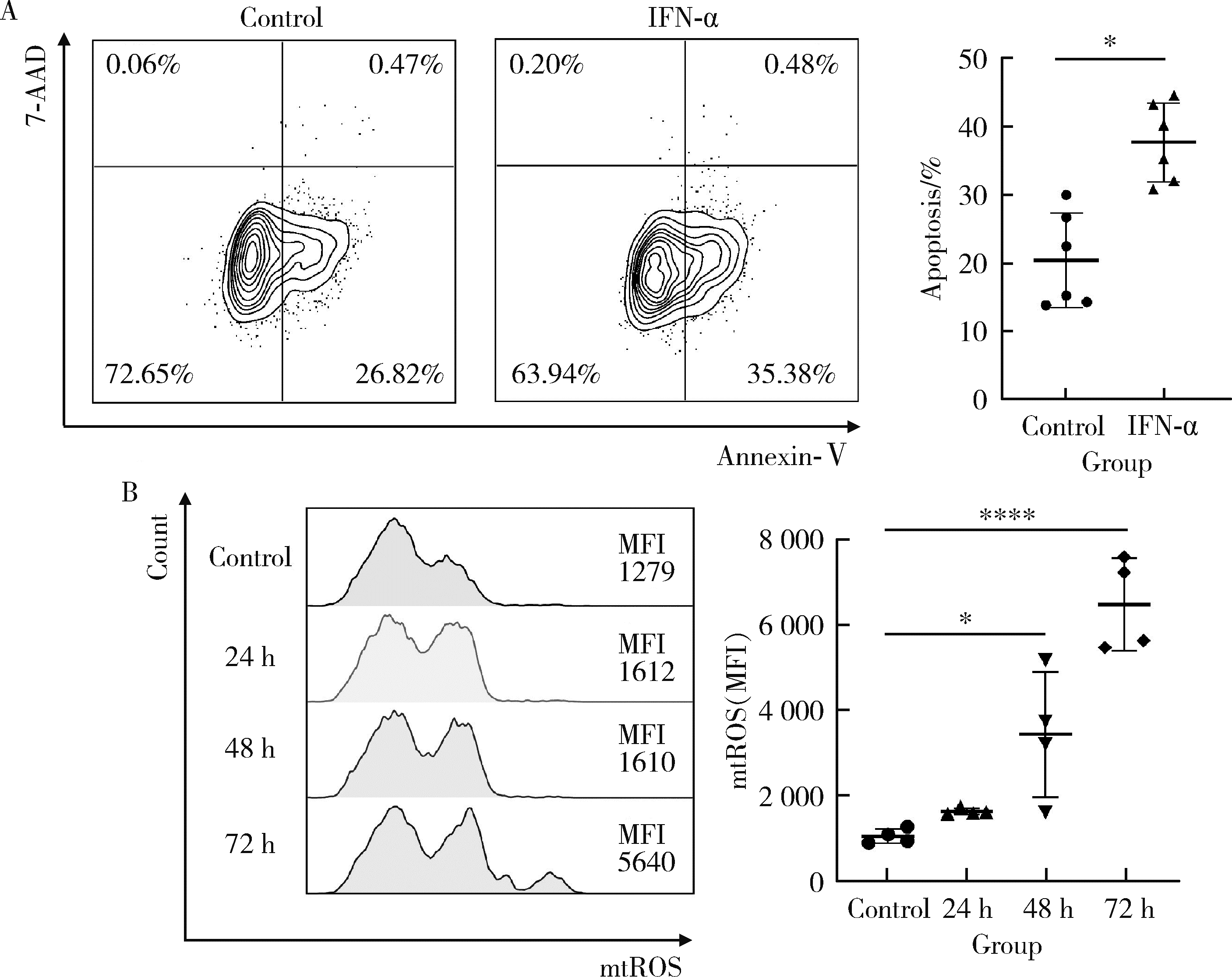
目的: 探究干扰素-α (interferon-α, IFN-α)对系统性红斑狼疮(systemic lupus erythematosus, SLE)患者外周血CD56dimCD57+自然杀伤(natural killer, NK)细胞凋亡和杀伤功能的调控作用及具体机制。方法: 选取大连医科大学附属第二医院就诊的64例初治SLE患者和16例健康人(healthy control, HC)为研究对象,采用实时荧光定量聚合酶链式反应检测NK细胞杀伤功能相关分子的基因表达水平;将CD56dimCD57+NK细胞与K562细胞共培养后,采用膜联蛋白和7氨基放线菌素标记凋亡的K562细胞;用浓度分别为20、40、80 μmol/L的过氧化氢(hydrogen peroxide, H2O2) 处理外周血单个核细胞,并以不使用H2O2处理作为对照组,采用流式细胞术检测穿孔素(per-forin, PRF)表达水平;采用酶联免疫吸附试验测定血清中IFN-α的浓度;采用流式细胞术检测CD56dimCD57+NK细胞表面IFN-α受体(interferon-α receptor, IFNAR)表达水平,并用平均荧光强度(mean fluorescence intensity, MFI)表示;用浓度为1 000 U/mL的IFN-α处理CD56dimCD57+NK细胞24、48、72 h,并以不使用IFN-α处理作为对照组,采用流式细胞术检测细胞凋亡及线粒体活性氧(mitochondrial reactive oxygen species, mtROS)表达水平,并用MFI表示。结果: 与HC(n=3)相比,SLE患者(n=3)外周血总NK细胞的PRF1基因表达水平降低(1.24±0.41 vs. 0.57±0.12, P=0.05)。与HC(n=5)相比,SLE患者(n=5)外周血CD56dimCD57+NK细胞杀伤K562细胞的能力明显下降(58.61%±10.60% vs. 36.74%±6.27%, P < 0.01)。与对照组(n=5, 97.51%±1.67%)相比,不同浓度的H2O2处理可显著下调CD56dimCD57+NK细胞的PRF表达水平且呈剂量依赖性,浓度为20 μmol/L的H2O2组PRF为83.23%±8.48% (n=5, P < 0.05),浓度为40 μmol/L的H2O2组PRF为79.53%±8.56% (n=5, P < 0.01),浓度为80 μmol/L的H2O2组PRF为76.67%±7.16% (n=5,P < 0.01)。与HC (n=16)相比,系统性红斑狼疮疾病活动评分(systemic lupus erythematosus disease activity index, SLEDAI) 为中高疾病活动度(SLEDAI≥10)的SLE患者(n=45)血清IFN-α水平显著增高[(55.07±50.36) ng/L vs. (328.2±276.3) ng/L, P < 0.001]。同时与HC (n=6)相比,SLE患者(n=6)外周血CD56dimCD57+NK细胞IFNAR1表达增高(MFI: 292.7±91.9 vs. 483.2±160.3, P < 0.05),与HC (n=6)相比,SLE患者(n=7)外周血CD56dimCD57+NK细胞IFNAR2表达增高(MFI: 643.5±113.7 vs. 919.0±246.9, P < 0.05)。与对照组(n=6)相比,IFN-α刺激(n=6)可显著促进CD56dimCD57+NK细胞凋亡(20.48%±7.01% vs. 37.82%±5.84%, P < 0.05)。同时,与对照组(n=4, MFI: 1 049±174.5)相比,不同时间下用IFN-α刺激CD56dimCD57+NK细胞可显著促进mtROS的产生,且呈时间依赖性,48 h的MFI为3 437±1 472 (n=4, P < 0.05),72 h的MFI为6 495±1 089 (n=4, P < 0.000 1),而刺激24 h时差异无统计学意义。结论: SLE患者血清中高水平的IFN-α可能通过促进mtROS产生诱导细胞凋亡并抑制PRF表达,下调CD56dimCD57+NK杀伤功能。
中图分类号:
- R593.2
| 1 |
Humbel M , Bellanger F , Fluder N , et al. Restoration of NK cell cytotoxic function with elotuzumab and daratumumab promotes elimination of circulating plasma cells in patients with SLE[J]. Front Immunol, 2021, 12, 645478.
doi: 10.3389/fimmu.2021.645478 |
| 2 |
Cheng Q , Chen X , Xu J , et al. lncRNA X-inactive-specific transcript is a potential biomarker related to changes in CD4+T cell levels in systemic lupus erythematosus[J]. Rheumatol Autoimmun, 2022, 2 (3): 159- 174.
doi: 10.1002/rai2.12048 |
| 3 | Lin SJ , Kuo ML , Hsiao HS , et al. Cytotoxic function and cytokine production of natural killer cells and natural killer T-like cells in systemic lupus erythematosis regulation with interleukin-15[J]. Mediators Inflamm, 2019, 2019, 4236562. |
| 4 |
Sordo-Bahamonde C , Lorenzo-Herrero S , Payer ÁR , et al. Mechanisms of apoptosis resistance to NK cell-mediated cytotoxicity in cancer[J]. Int J Mol Sci, 2020, 21 (10): 3726.
doi: 10.3390/ijms21103726 |
| 5 | Lorenzo-Herrero S , Sordo-Bahamonde C , Gonzalez S , et al. CD107a degranulation assay to evaluate immune cell antitumor activity[J]. Methods Mol Biol, 2019, 1884, 119- 130. |
| 6 |
Myers JA , Miller JS . Exploring the NK cell platform for cancer immunotherapy[J]. Nat Rev Clin Oncol, 2021, 18 (2): 85- 100.
doi: 10.1038/s41571-020-0426-7 |
| 7 |
Quatrini L , Della Chiesa M , Sivori S , et al. Human NK cells, their receptors and function[J]. Eur J Immunol, 2021, 51 (7): 1566- 1579.
doi: 10.1002/eji.202049028 |
| 8 |
Kucuksezer UC , Aktas Cetin E , Esen F , et al. The role of natural killer cells in autoimmune diseases[J]. Front Immunol, 2021, 12, 622306.
doi: 10.3389/fimmu.2021.622306 |
| 9 |
Bryceson YT , March ME , Barber DF , et al. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells[J]. J Exp Med, 2005, 202 (7): 1001- 1012.
doi: 10.1084/jem.20051143 |
| 10 | Nielsen CM , White MJ , Goodier MR , et al. Functional significance of CD57 expression on human NK cells and relevance to disease[J]. Front Immunol, 2013, 4, 422. |
| 11 |
Lu Z , Tian Y , Bai Z , et al. Increased oxidative stress contributes to impaired peripheral CD56dimCD57+ NK cells from patients with systemic lupus erythematosus[J]. Arthritis Res Ther, 2022, 24 (1): 48.
doi: 10.1186/s13075-022-02731-y |
| 12 |
Banchereau R , Hong S , Cantarel B , et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients[J]. Cell, 2016, 165 (3): 551- 565.
doi: 10.1016/j.cell.2016.03.008 |
| 13 |
Li P , Jiang M , Li K , et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity[J]. Nat Immunol, 2021, 22 (9): 1107- 1117.
doi: 10.1038/s41590-021-00993-3 |
| 14 |
Furie R , Werth VP , Merola JF , et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus[J]. J Clin Invest, 2019, 129 (3): 1359- 1371.
doi: 10.1172/JCI124466 |
| 15 |
Chatham WW , Furie R , Saxena A , et al. Long-term safety and efficacy of anifrolumab in adults with systemic lupus erythematosus: Results of a phase Ⅱ open-label extension study[J]. Arthritis Rheumatol, 2021, 73 (5): 816- 825.
doi: 10.1002/art.41598 |
| 16 |
Kirou KA , Mavragani CP , Crow MK . Activation of type I inter-feron in systemic lupus erythematosus[J]. Expert Rev Clin Immunol, 2007, 3 (4): 579- 588.
doi: 10.1586/1744666X.3.4.579 |
| 17 |
Le Bon A , Thompson C , Kamphuis E , et al. Cutting edge: Enhancement of antibody responses through direct stimulation of B and T cells by type I IFN[J]. J Immunol, 2006, 176 (4): 2074- 2078.
doi: 10.4049/jimmunol.176.4.2074 |
| 18 |
Cucak H , Yrlid U , Reizis B , et al. Type Ⅰ interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells[J]. Immunity, 2009, 31 (3): 491- 501.
doi: 10.1016/j.immuni.2009.07.005 |
| 19 | De Groof A , Ducreux J , Aleva F , et al. STAT3 phosphorylation mediates the stimulatory effects of interferon alpha on B cell dif-ferentiation and activation in SLE[J]. Rheumatology (Oxford), 2020, 59 (3): 668- 677. |
| 20 | Hochberg MC . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997, 40 (9): 1725. |
| 21 | Li H , Geng L , Cao Z , et al. CD56brightCD16- to CD57+CD56dimCD16+ NK cell ratio discriminates disease activity and renal involvement in patients with systemic lupus erythematosus[J]. Clin Exp Rheumatol, 2023, 41 (9): 1768- 1776. |
| 22 |
Yang Y , Day J , Souza-Fonseca Guimaraes F , et al. Natural killer cells in inflammatory autoimmune diseases[J]. Clin Transl Immunology, 2021, 10 (2): e1250.
doi: 10.1002/cti2.1250 |
| 23 |
Streltsova MA , Erokhina SA , Kanevskiy LM , et al. Analysis of NK cell clones obtained using interleukin-2 and gene-modified K562 cells revealed the ability of "senescent" NK cells to lose CD57 expression and start expressing NKG2A[J]. PLoS One, 2018, 13 (12): e0208469.
doi: 10.1371/journal.pone.0208469 |
| 24 | Bahadorian D , Mollazadeh S , Mirazi H , et al. Regulatory NK cells in autoimmune disease[J]. Iran J Basic Med Sci, 2023, 26 (6): 609- 616. |
| 25 |
Fresneda Alarcon M , McLaren Z , Wright HL . Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same foe different M.O[J]. Front Immunol, 2021, 12, 649693.
doi: 10.3389/fimmu.2021.649693 |
| 26 |
Shah D , Mahajan N , Sah S , et al. Oxidative stress and its biomarkers in systemic lupus erythematosus[J]. J Biomed Sci, 2014, 21 (1): 23.
doi: 10.1186/1423-0127-21-23 |
| 27 |
Kennel KB , Greten FR . Immune cell-produced ROS and their impact on tumor growth and metastasis[J]. Redox Biol, 2021, 42, 101891.
doi: 10.1016/j.redox.2021.101891 |
| 28 |
Song H , Park H , Kim YS , et al. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species[J]. Int Immunopharmacol, 2011, 11 (8): 932- 938.
doi: 10.1016/j.intimp.2011.02.005 |
| 29 |
He H , Wang C , Liu G , et al. Isobavachalcone inhibits acute myeloid leukemia: Potential role for ROS-dependent mitochondrial apoptosis and differentiation[J]. Phytother Res, 2021, 35 (6): 3337- 3350.
doi: 10.1002/ptr.7054 |
| 30 |
Bhattacharya A , Hegazy AN , Deigendesch N , et al. Superoxide dismutase 1 protects hepatocytes from type I interferon-driven oxidative damage[J]. Immunity, 2015, 43 (5): 974- 986.
doi: 10.1016/j.immuni.2015.10.013 |
| 31 |
Tasdogan A , Kumar S , Allies G , et al. DNA damage-induced HSPC malfunction depends on ROS accumulation downstream of IFN-1 signaling and bid mobilization[J]. Cell Stem Cell, 2016, 19 (6): 752- 767.
doi: 10.1016/j.stem.2016.08.007 |
| 32 |
Buang N , Tapeng L , Gray V , et al. Type Ⅰ interferons affect the metabolic fitness of CD8+ T cells from patients with systemic lupus erythematosus[J]. Nat Commun, 2021, 12 (1): 980.
doi: 10.1038/s41467-021-21210-7 |
| [1] | 田佳宜, 郭一学, 张霞, 孙晓麟, 何菁. 中国健康成人外周血自然杀伤细胞及其亚群的正常值范围流式细胞学分析[J]. 北京大学学报(医学版), 2024, 56(5): 839-844. |
| [2] | 武志慧, 胡明智, 赵巧英, 吕凤凤, 张晶莹, 张伟, 王永福, 孙晓林, 王慧. miR-125b-5p修饰脐带间充质干细胞对系统性红斑狼疮的免疫调控机制[J]. 北京大学学报(医学版), 2024, 56(5): 860-867. |
| [3] | 乔佳佳,田聪,黄晓波,刘军. 肾结石合并系统性红斑狼疮行经皮肾镜碎石取石术的安全性和有效性评估[J]. 北京大学学报(医学版), 2024, 56(4): 745-749. |
| [4] | 任立敏,赵楚楚,赵义,周惠琼,张莉芸,王友莲,沈凌汛,范文强,李洋,厉小梅,王吉波,程永静,彭嘉婧,赵晓珍,邵苗,李茹. 系统性红斑狼疮低疾病活动度及缓解状况的真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 273-278. |
| [5] | 罗芷筠,吴佳佳,宋优,梅春丽,杜戎. 伴神经精神系统病变的系统性红斑狼疮相关巨噬细胞活化综合征2例[J]. 北京大学学报(医学版), 2023, 55(6): 1111-1117. |
| [6] | 姚海红,杨帆,唐素玫,张霞,何菁,贾园. 系统性红斑狼疮及成人Still病合并巨噬细胞活化综合征的临床特点及诊断指标[J]. 北京大学学报(医学版), 2023, 55(6): 966-974. |
| [7] | 邵苗,郭惠芳,雷玲彦,赵清,丁艳杰,林进,吴锐,于峰,李玉翠,苗华丽,张莉芸,杜燕,焦瑞英,庞丽霞,龙丽,栗占国,李茹. 短间期小剂量环磷酰胺治疗系统性红斑狼疮耐受性的多中心对照研究[J]. 北京大学学报(医学版), 2022, 54(6): 1112-1116. |
| [8] | 李敏,侯林卿,金月波,何菁. 系统性红斑狼疮合并视网膜病变的临床及免疫学特点[J]. 北京大学学报(医学版), 2022, 54(6): 1106-1111. |
| [9] | 张琳崎,赵静,王红彦,王宗沂,李英妮,汤稷旸,李思莹,曲进锋,赵明威. 抗ENO1抗体与狼疮性视网膜病变的相关性[J]. 北京大学学报(医学版), 2022, 54(6): 1099-1105. |
| [10] | 梁秀睿,闪雪纯,关晶,张锐,杨静,张怡,金佳琦,张誉馨,徐凡,傅继华. 高血糖诱导肝星状细胞5-羟色胺降解在2型糖尿病致肝脏炎症和纤维化时的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1141-1150. |
| [11] | 邹健梅,武丽君,罗采南,石亚妹,吴雪. 血清25-羟维生素D与系统性红斑狼疮活动的关系[J]. 北京大学学报(医学版), 2021, 53(5): 938-941. |
| [12] | 马向波,张学武,贾汝琳,高颖,刘洪江,刘玉芳,李英妮. 外周血淋巴细胞亚群检测在系统性硬化症治疗中的应用[J]. 北京大学学报(医学版), 2021, 53(4): 721-727. |
| [13] | 夏芳芳,鲁芙爱,吕慧敏,杨国安,刘媛. 系统性红斑狼疮伴间质性肺炎的临床特点及相关因素分析[J]. 北京大学学报(医学版), 2021, 53(2): 266-272. |
| [14] | 耿研,李伯睿,张卓莉. 系统性红斑狼疮患者有症状关节病变的肌肉骨骼超声特点[J]. 北京大学学报(医学版), 2020, 52(1): 163-168. |
| [15] | 王玉华,张国华,张令令,罗俊丽,高兰. 系统性红斑狼疮合并自发性肾上腺出血1例[J]. 北京大学学报(医学版), 2019, 51(6): 1178-1181. |
|
||