北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (6): 950-955. doi: 10.19723/j.issn.1671-167X.2024.06.002
铁死亡标志物4-HNE在系统性硬化症细胞模型中的表达及意义
赵柯林1, 夏雪1, 史乃旭2, 周韩1, 盖婧雯1, 李萍1,*( )
)
- 1. 吉林大学中日联谊医院风湿免疫科,长春 130000
2. 吉林大学中日联谊医院口腔科,长春 130000
Expression and significance of ferroptosis marker 4-HNE in in vitro model of systemic sclerosis
Kelin ZHAO1, Xue XIA1, Naixu SHI2, Han ZHOU1, Jingwen GAI1, Ping LI1,*( )
)
- 1. Department of Rheumatology and Immunology, China-Japan Union Hospital, Jilin University, Changchun 130000, China
2. Department of Stomatology, China-Japan Union Hospital, Jilin University, Changchun 130000, China
摘要:
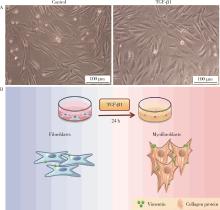
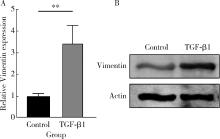
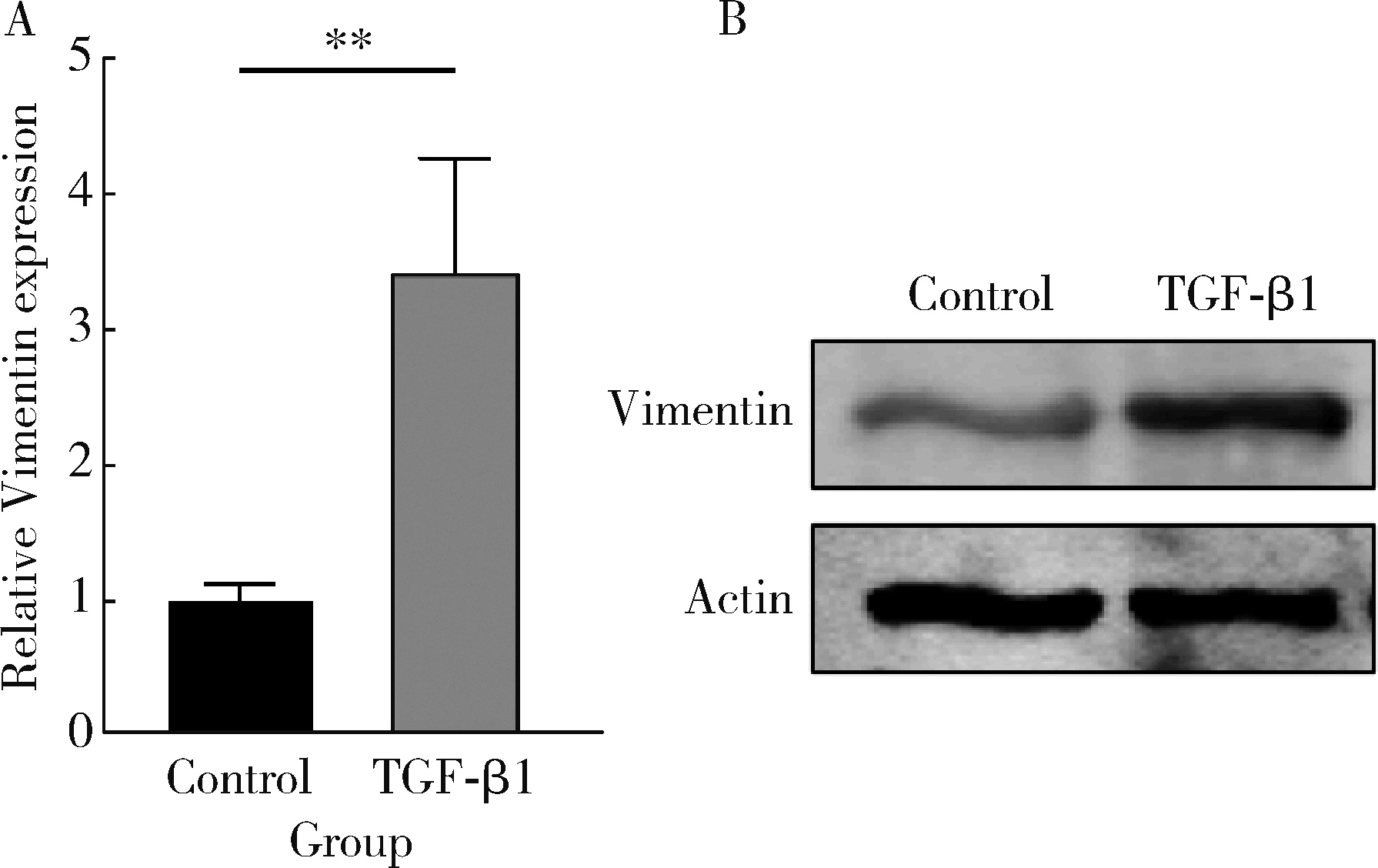
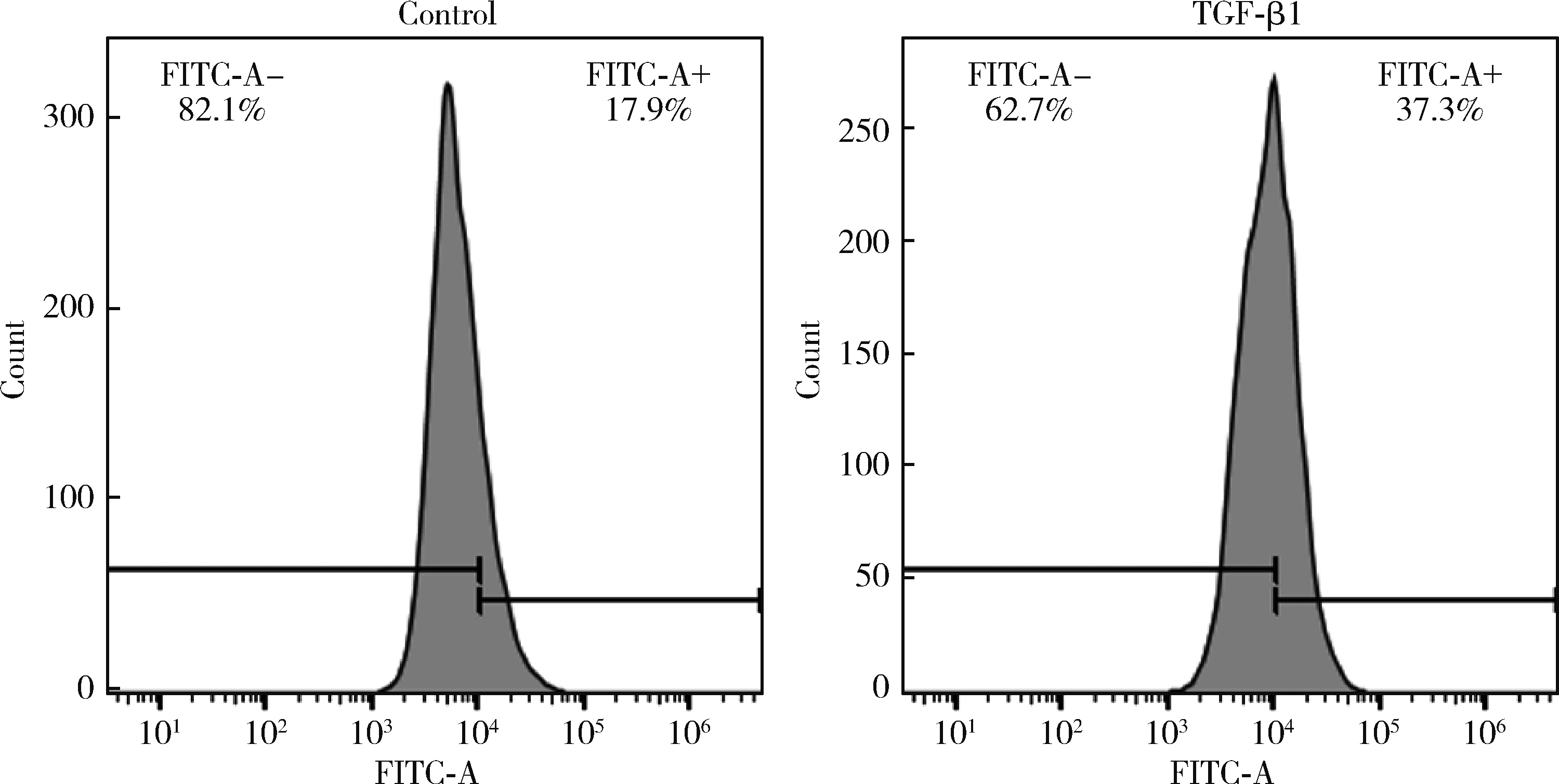
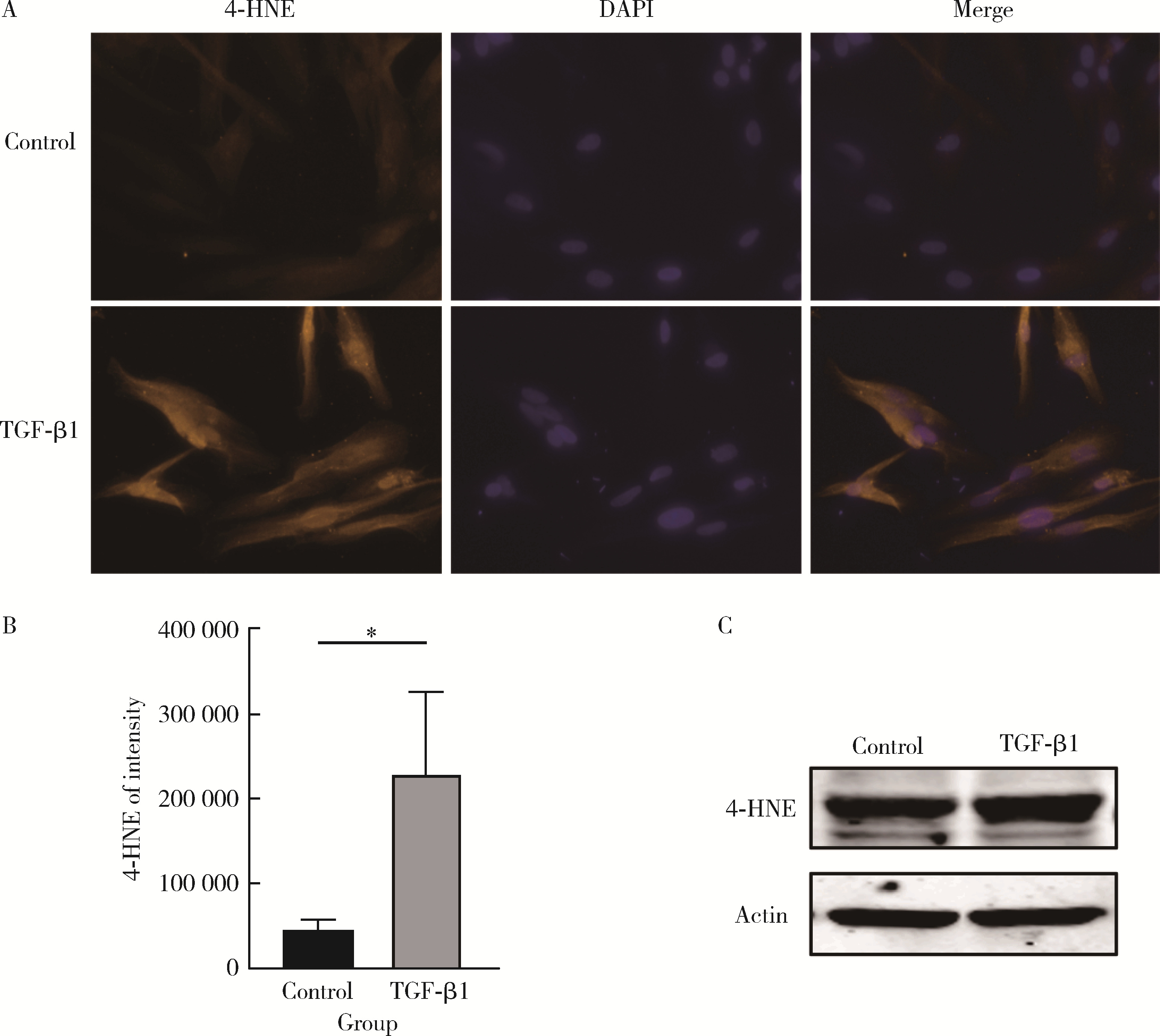
目的: 探究铁死亡标志物4-羟基壬烯醛(4-hydroxynonenal, 4-HNE)在转化生长因子β1(transforming growth factor-beta 1, TGF-β1)诱导的肌成纤维细胞模型中的表达及其生理意义,为系统性硬化症(systemic sclerosis, SSc)纤维化进程的诊断和治疗提供理论依据。方法: 将饥饿处理12 h后的小鼠胚胎成纤维细胞(NIH3t3)分为两组,对照组以1%(体积分数)血清培养基培养,TGF-β1处理组以10 μg/L TGF-β1+1%血清培养基培养。通过显微镜观察两组细胞形态的变化,使用实时荧光定量逆转录PCR(reverse transcription quantitative real-time PCR, RT-qPCR)和蛋白免疫印迹实验(Western blot)检测纤维化标志物的表达,验证SSc细胞模型的构建。使用流式细胞术分析两组细胞内活性氧(reactive oxygen species, ROS)的水平,通过Western blot和免疫荧光染色检测TGF-β1处理组中4-HNE的表达水平。结果: 显微镜下观察到TGF-β1处理的NIH3t3细胞形态从典型的长梭形逐渐转变为多突起的扁平三角形。RT-qPCR和Western blot检测结果表明,TGF-β1组中纤维化标志物波形蛋白(Vimentin)的表达显著高于对照组(P<0.01),证实了TGF-β1可以促进纤维化标志物的上调。流式细胞术结果显示,TGF-β1处理组中细胞内ROS水平显著升高,表明其诱导了氧化应激的发生。Western blot和免疫荧光分析均显示,TGF-β1处理后细胞中4-HNE的表达显著增加(免疫荧光强度P<0.05)。结论: TGF-β1可诱导成纤维细胞发生纤维化,同时促进ROS的生成,并显著上调4-HNE的表达水平;4-HNE的显著增加提示其在SSc纤维化过程中可能具有重要作用,可作为潜在的纤维化标志物;本研究为未来探讨4-HNE在SSc中的作用机制和其作为诊断和治疗靶点的可行性提供了实验依据。
中图分类号:
- R593.25
| 1 |
Hinz B , Phan SH , Thannickal VJ , et al. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling[J]. Am J Pathol, 2012, 180 (4): 1340- 1355.
doi: 10.1016/j.ajpath.2012.02.004 |
| 2 |
Volkmann ER , Andreasson K , Smith V . Systemic sclerosis[J]. Lancet, 2023, 401 (10373): 304- 318.
doi: 10.1016/S0140-6736(22)01692-0 |
| 3 |
Preliminary criteria for the classification of systemic sclerosis (scleroderma) . Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee[J]. Arthritis Rheum, 1980, 23 (5): 581- 590.
doi: 10.1002/art.1780230510 |
| 4 | Leroy EC , Medsger TA Jr . Criteria for the classification of early systemic sclerosis[J]. J Rheumatol, 2001, 28 (7): 1573- 1576. |
| 5 |
Vona R , Giovannetti A , Gambardella L , et al. Oxidative stress in the pathogenesis of systemic scleroderma: An overview[J]. J Cell Mol Med, 2018, 22 (7): 3308- 3314.
doi: 10.1111/jcmm.13630 |
| 6 |
Doridot L , Jeljeli M , Chene C , et al. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis[J]. Redox Biol, 2019, 25, 101122.
doi: 10.1016/j.redox.2019.101122 |
| 7 |
Doll S , Proneth B , Tyurina YY , et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition[J]. Nat Chem Biol, 2017, 13 (1): 91- 98.
doi: 10.1038/nchembio.2239 |
| 8 |
Cao D , Zheng J , Li Z , et al. ACSL4 inhibition prevents macrophage ferroptosis and alleviates fibrosis in bleomycin-induced systemic sclerosis model[J]. Arthritis Res Ther, 2023, 25 (1): 212.
doi: 10.1186/s13075-023-03190-9 |
| 9 |
Dalleau S , Baradat M , Gueraud F , et al. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance[J]. Cell Death Differ, 2013, 20 (12): 1615- 1630.
doi: 10.1038/cdd.2013.138 |
| 10 |
Galam L , Failla A , Soundararajan R , et al. 4-hydroxynonenal regulates mitochondrial function in human small airway epithelial cells[J]. Oncotarget, 2015, 6 (39): 41508- 41521.
doi: 10.18632/oncotarget.6131 |
| 11 |
Reyes-Jimenez E , Ramirez-Hernandez AA , Santos-Alvarez JC , et al. Coadministration of 3'5-dimaleamylbenzoic acid and quercetin decrease pulmonary fibrosis in a systemic sclerosis model[J]. Int Immunopharmacol, 2023, 122, 110664.
doi: 10.1016/j.intimp.2023.110664 |
| 12 |
de Virgilio A , Greco A , Fabbrini G , et al. Parkinson ' s disease: Autoimmunity and neuroinflammation[J]. Autoimmun Rev, 2016, 15 (10): 1005- 1011.
doi: 10.1016/j.autrev.2016.07.022 |
| 13 | Li Y , Zhao T , Li J , et al. Oxidative stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the pathogenesis and treatment of aging-related diseases[J]. J Immunol Res, 2022, 2022, 2233906. |
| 14 |
Rozier P , Maumus M , Bony C , et al. Extracellular vesicles are more potent than adipose mesenchymal stromal cells to exert an anti-fibrotic effect in an in vitro model of systemic sclerosis[J]. Int J Mol Sci, 2021, 22 (13): 6837.
doi: 10.3390/ijms22136837 |
| 15 |
Wu C , Liu J , Chen Z , et al. Comprehensive analysis of ferroptosis-related hub gene signatures as a potential pathogenesis and therapeutic target for systemic sclerosis: A bioinformatics analysis[J]. Int J Immunopathol Pharmacol, 2023, 37, 3946320231187783.
doi: 10.1177/03946320231187783 |
| 16 |
Pei Z , Qin Y , Fu X , et al. Inhibition of ferroptosis and iron accumulation alleviates pulmonary fibrosis in a bleomycin model[J]. Redox Biol, 2022, 57, 102509.
doi: 10.1016/j.redox.2022.102509 |
| 17 |
Wu J , Feng Z , Chen L , et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models[J]. Nat Commun, 2022, 13 (1): 676.
doi: 10.1038/s41467-021-27948-4 |
| 18 |
Luczaj W , Gindzienska-Sieskiewicz E , Jarocka-Karpowicz I , et al. The onset of lipid peroxidation in rheumatoid arthritis: Consequences and monitoring[J]. Free Radic Res, 2016, 50 (3): 304- 313.
doi: 10.3109/10715762.2015.1112901 |
| 19 |
Chen J , Chen P , Song Y , et al. STING upregulation mediates ferroptosis and inflammatory response in lupus nephritis by upregu-lating TBK1 and activating NF-kappaB signal pathway[J]. J Biosci, 2024, 49, 9.
doi: 10.1007/s12038-023-00381-z |
| 20 |
Brezovec N , Perdan-Pirkmajer K , Burja B , et al. Disturbed antioxidant capacity in patients with systemic sclerosis associates with lung and gastrointestinal symptoms[J]. Biomedicines, 2023, 11 (8): 2110.
doi: 10.3390/biomedicines11082110 |
| 21 |
Varga J , Pasche B . Transforming growth factor beta as a therapeutic target in systemic sclerosis[J]. Nat Rev Rheumatol, 2009, 5 (4): 200- 206.
doi: 10.1038/nrrheum.2009.26 |
| 22 |
Piera-Velazquez S , Makul A , Jimenez SA . Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: Regulation by transforming growth factor beta[J]. Arthritis Rheumatol, 2015, 67 (10): 2749- 2758.
doi: 10.1002/art.39242 |
| 23 |
Jimenez SA , Gaidarova S , Saitta B , et al. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts[J]. J Clin Invest, 2001, 108 (9): 1395- 1403.
doi: 10.1172/JCI200112347 |
| 24 |
Sun X , Majumder P , Shioya H , et al. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species[J]. J Biol Chem, 2000, 275 (23): 17237- 17240.
doi: 10.1074/jbc.C000099200 |
| 25 |
Svegliati S , Spadoni T , Moroncini G , et al. NADPH oxidase, oxidative stress and fibrosis in systemic sclerosis[J]. Free Radic Biol Med, 2018, 125, 90- 97.
doi: 10.1016/j.freeradbiomed.2018.04.554 |
| 26 |
Zhou X , Trinh-Minh T , Tran-Manh C , et al. Impaired mitochondrial transcription factor A expression promotes mitochondrial damage to drive fibroblast activation and fibrosis in systemic sclerosis[J]. Arthritis Rheumatol, 2022, 74 (5): 871- 881.
doi: 10.1002/art.42033 |
| 27 |
Liu W , Porter NA , Schneider C , et al. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition[J]. Free Radic Biol Med, 2011, 50 (1): 166- 178.
doi: 10.1016/j.freeradbiomed.2010.10.709 |
| 28 |
Yang HJ , Hu R , Sun H , et al. 4-HNE induces proinflammatory cytokines of human retinal pigment epithelial cells by promoting extracellular efflux of HSP70[J]. Exp Eye Res, 2019, 188, 107792.
doi: 10.1016/j.exer.2019.107792 |
| [1] | 潘苇, 李云, 罗俊佳, 李春, 叶华, 李雪, 贾园. 系统性硬化症患者新型冠状病毒感染特点及疫苗接种效果:一项单中心队列研究[J]. 北京大学学报(医学版), 2024, 56(6): 1041-1046. |
| [2] | 李炳乐, 朱凌妍, 王永福, 白力. 褪黑素调控节律基因表达及其缓解间质性肺纤维化的机制[J]. 北京大学学报(医学版), 2024, 56(6): 963-971. |
| [3] | 汤莹, 张湧波, 吴丹红, 林炎鸿, 兰风华. 13例先天性双侧输精管缺如不育患者的致病基因突变检测[J]. 北京大学学报(医学版), 2024, 56(5): 763-774. |
| [4] | 何珊,陈炘,程琦,朱灵江,张培玉,童淑婷,薛静,杜燕. 托法替布通过JAK/STAT3通路抑制肺成纤维细胞向肌成纤维细胞转化[J]. 北京大学学报(医学版), 2024, 56(3): 505-511. |
| [5] | 李文根,古晓东,翁锐强,刘苏东,陈超. 血浆外泌体miR-34-5p和miR-142-3p在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2023, 55(6): 1022-1027. |
| [6] | 王磊,金香淑,董慧君,欧国敏,赖鑫源,庄辉,李彤,向宽辉. 基于COL1A1启动子和增强型绿色荧光蛋白基因建立人肝星状细胞活化的细胞模型[J]. 北京大学学报(医学版), 2023, 55(5): 876-885. |
| [7] | 林卓华,蔡如意,孙洋,穆荣,崔立刚. 超微血流显像评价系统性硬化症指端血流的方法学与临床应用[J]. 北京大学学报(医学版), 2023, 55(4): 636-640. |
| [8] | 梁秀睿,闪雪纯,关晶,张锐,杨静,张怡,金佳琦,张誉馨,徐凡,傅继华. 高血糖诱导肝星状细胞5-羟色胺降解在2型糖尿病致肝脏炎症和纤维化时的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1141-1150. |
| [9] | 罗靓,蔡青猛,刘香君,贠泽霖,李春,张晓盈. 以雷诺现象为首发表现的系统性硬化症临床特征及其相关因素[J]. 北京大学学报(医学版), 2022, 54(6): 1224-1228. |
| [10] | 蔡天玉,朱振鹏,徐纯如,吉星,吕同德,郭振可,林健. 成纤维细胞生长因子受体2在肾透明细胞癌中的表达及意义[J]. 北京大学学报(医学版), 2022, 54(4): 628-635. |
| [11] | 马向波,张学武,贾汝琳,高颖,刘洪江,刘玉芳,李英妮. 外周血淋巴细胞亚群检测在系统性硬化症治疗中的应用[J]. 北京大学学报(医学版), 2021, 53(4): 721-727. |
| [12] | 郜洪宇,孟焕新,侯建霞,黄宝鑫,李玮. 钙结合蛋白在健康牙周组织和实验性牙周炎组织的表达分布[J]. 北京大学学报(医学版), 2021, 53(4): 744-749. |
| [13] | 赵静,孙峰,李云,赵晓珍,徐丹,李英妮,李玉慧,孙晓麟. 抗α-1C微管蛋白抗体在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2020, 52(6): 1009-1013. |
| [14] | 刘世博,高辉,冯元春,李静,张彤,万利,刘燕鹰,李胜光,罗成华,张学武. 腹膜后纤维化致肾盂积水的临床分析:附17例报道[J]. 北京大学学报(医学版), 2020, 52(6): 1069-1074. |
| [15] | 石冰清,袁晓静,赵玉鸣. 比较矿物三氧化物凝聚体及山东蜂胶乙醇提取物对牙髓成纤维细胞生物学性能的影响[J]. 北京大学学报(医学版), 2019, 51(6): 1108-1114. |
|
||