北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (6): 989-995. doi: 10.19723/j.issn.1671-167X.2019.06.002
免疫介导坏死性肌病的临床和病理特征分析
杨红霞1,2,田小兰2,江薇2,李文丽2,刘青艳2,彭清林2,王国春2,卢昕1,△( )
)
- 1. 北京大学中日友好临床医学院,北京 100029
2. 中日友好医院风湿免疫科,北京 100029
Clinical and pathological characteristics of immune mediated necrotizing myopathy
Hong-xia YANG1,2,Xiao-lan TIAN2,Wei JIANG2,Wen-li LI2,Qing-yan LIU2,Qing-lin PENG2,Guo-chun WANG2,Xin LU1,△( )
)
- 1. Peking University China-Japan Friendship School of Clinical Medicine, Beijing 100029, China
2. Department of Rheumatology, China-Japan Friendship Hospital, Beijing 100029, China
摘要:
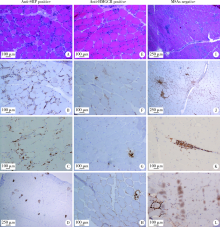
目的 比较不同肌炎特异性自身抗体(myositis specific antibodies,MSAs)类型的免疫介导坏死性肌病(immune-mediated necrotizing myopathies,IMNM)的临床和病理特征。方法 从中日友好医院2008—2018年住院期间所有行肌肉活检的特发性炎性肌病患者中选取符合以下任一条件的IMNM患者104例:(1)抗信号识别颗粒(signal recognition particle,SRP)抗体阳性;(2)抗3-羟基-3-甲基戊二酰辅酶A 还原酶(3-hydroxy-3-methylglutaryl-coenzyme A reductase,HMGCR)抗体阳性;(3)血清MSAs阴性且病理符合2004年欧洲神经肌肉病中心定义的IMNM病理诊断标准。回顾性收集患者的临床、实验室检查和肌肉病理等信息,比较各组临床及病理特征的差异。结果 所有104例IMNM患者中,肌无力(92.3%)、肌酸激酶升高(92.3%)是IMNM的最常见临床表现,此外,33.7%的IMNM患者合并吞咽困难,46.5%的患者合并间质性肺病(interstitial lung diseases,ILD)。抗HMGCR阳性患者较抗SRP阳性和MSAs阴性患者更容易出现V形疹(30.4% vs. 4.3%和5.9%,P<0.01),抗SRP阳性患者合并ILD发生率高于抗HMGCR阳性和MSAs阴性患者(64.4% vs. 34.8%和29.0%,P<0.01),MSAs阴性患者合并其他结缔组织病更多见(32.4% vs. 8.5%和4.3%,P<0.01)。3组IMNM患者肌肉病理中均可见肌细胞坏死(94.2%)、吞噬(65.4%)和再生(67.3%),肌细胞膜表达主要组织相容性复合物-Ⅰ分子上调(78.8%),肌内膜CD4 +T细胞(68.3%)和CD68 +巨噬细胞(65.7%)浸润。结论 抗SRP抗体阳性、抗HMGCR抗体阳性和MSAs阴性的IMNM患者存在异质性,在临床上开展MSAs检测和肌肉病理检查对区分不同类型的IMNM有指导价值。
中图分类号:
- R593.26
| [1] | Hoogendijk JE, Amato AA, Lecky BR , et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies,with the exception of inclusion body myositis[J]. Neuromuscul Disord, 2004,14(5):337-345. |
| [2] | Allenbach Y, Mammen AL, Benveniste O , et al. 224th ENMC International Workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies[J]. Neuromuscul Disord, 2018,28(1):87-99. |
| [3] | Pinal-Fernandez I, Mammen AL . Spectrum of immune-mediated necrotizing myopathies and their treatments[J]. Curr Opin Rheumatol, 2016,28(6):619-624. |
| [4] | Lim J, Rietveld A, De Bleecker JL , et al. Seronegative patients form a distinctive subgroup of immune-mediated necrotizing myopathy[J]. Neurol Neuroimmunol Neuroinflamm, 2018,6(1):e513. |
| [5] | Bohan A, Peter JB . Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975,292(7):344-347. |
| [6] | Bohan A, Peter JB . Polymyositis and dermatomyositis (second of two parts)[J]. N Engl J Med, 1975,292(8):403-407. |
| [7] | Suzuki S, Nishikawa A, Kuwana M , et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients[J]. Orphanet J Rare Dis, 2015,5(13):10-61. |
| [8] | Suzuki S, Hayashi YK, Kuwana M , et al. Myopathy associated with anti- bodies to signal recognition particle: disease progression and neurological outcome[J]. Arch Neurol, 2012,69(6):728-732. |
| [9] | Raghu G, Collard HR, Egan JJ , et al. An official ATSERSJRSALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management[J]. Am J Respir Crit Care Med, 2011,183(6):788-824. |
| [10] | Travis WD, Costabel U, Hansell DM , et al. An official American Thoracic Society European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias[J]. Am J Respir Crit Care Med, 2013,188(6):733-748. |
| [11] | Dalakas MC . Polymyositis, dermatomyositis and inclusion-body myositis[J]. N Engl J Med, 1991,325(21):1487-1498. |
| [12] | Watanabe Y, Uruha A, Suzuki S , et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy[J]. Neurol Neurosurg Psychiatry, 2016,87(10):1038-1044. |
| [13] | Takada T, Hirakata M, Suwa A , et al. Clinical and histopatholo-gical features of myopathies in Japanese patients with anti-SRP autoantibodies[J]. Mod Rheumatol, 2009,19(2):156-164. |
| [14] | Ge Y, Lu X, Peng Q , et al. Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibodies in Chinese patients with idiopathic inflammatory myopathies[J]. PLoS One, 2015,10(10):e0141616. |
| [15] | Limaye V, Bundell C, Hollingsworth P , et al. Clinical and gene-tic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy[J]. Muscle Nerve, 2015,52(2):196-203. |
| [16] | Christopher-Stine L, Casciola-Rosen LA, Hong G , et al. A novel autoantibody recognizing 200-kD and 100-kD proteins is associated with an immune-mediated necrotizing myopathy[J]. Arthritis Rheum, 2010,62(9):2757-2766. |
| [17] | Mammens AL . Which nonautoimmune myopathies are most frequently misdiagnosed as myositis?[J]. Curr Opin Rheumatol, 2017,29(6):618-622. |
| [18] | Wang Q, Li Y, Ji SQ , et al. Immunopathological characterization of muscle biopsy samples from immune-mediated necrotizing myo-pathy patients[J]. Med Sci Monit, 2018,12(24):2189-2196. |
| [19] | Wang L, Liu LL, Hao HJ , et al. Myopathy with anti-signal recognition particle antibodies: clinical and histopathological features in Chinese patients[J]. Neuromuscular Disorders, 2014,24(4):335-341. |
| [1] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [2] | 李挺. 建设当代临床病理学科[J]. 北京大学学报(医学版), 2023, 55(2): 197-200. |
| [3] | 周桥. 肿瘤病理学研究的进展和展望[J]. 北京大学学报(医学版), 2023, 55(2): 201-209. |
| [4] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
| [5] | 侯卫华,宋书杰,石中月,金木兰. 幽门螺杆菌阴性早期胃癌的临床病理特征[J]. 北京大学学报(医学版), 2023, 55(2): 292-298. |
| [6] | 刘菊梅,梁丽,张继新,戎龙,张梓怡,吴悠,赵旭东,李挺. 411例早期胃癌及癌前病变内镜黏膜下剥离术标本的病理学评估[J]. 北京大学学报(医学版), 2023, 55(2): 299-307. |
| [7] | 农琳,王微,梁丽,李东,李鑫,李挺. 母细胞性浆样树突细胞肿瘤13例临床病理学特征[J]. 北京大学学报(医学版), 2023, 55(2): 308-314. |
| [8] | 熊焰,张波,聂立功,吴世凯,赵虎,李东,邸吉廷. 胸部SMARCA4缺失性未分化肿瘤的病理诊断与联合免疫检测点抑制剂治疗[J]. 北京大学学报(医学版), 2023, 55(2): 351-356. |
| [9] | 哈雪梅,姚永正,孙莉华,辛春杨,熊焰. 实性肺胎盘样变形1例及文献复习[J]. 北京大学学报(医学版), 2023, 55(2): 357-361. |
| [10] | 宁博涵,张青霞,杨慧,董颖. 伴间质细胞增生、玻璃样变性及索状结构的子宫内膜样腺癌1例[J]. 北京大学学报(医学版), 2023, 55(2): 366-369. |
| [11] | 邢晓燕,张筠肖,朱冯赟智,王一帆,周新尧,李玉慧. 皮肌炎合并巨噬细胞活化综合征5例[J]. 北京大学学报(医学版), 2022, 54(6): 1214-1218. |
| [12] | 博尔术,洪鹏,张宇,邓绍晖,葛力源,陆敏,李楠,马潞林,张树栋. 乳头状肾细胞癌的临床病理特征和预后分析[J]. 北京大学学报(医学版), 2022, 54(4): 615-620. |
| [13] | 应沂岑,唐琦,杨恺惟,米悦,范宇,虞巍,宋毅,何志嵩,周利群,李学松. 泌尿肿瘤免疫检查点抑制剂相关性肌炎的临床特征[J]. 北京大学学报(医学版), 2022, 54(4): 644-651. |
| [14] | 李炳雨,唐祖南,胡耒豪,章文博,于尧,俞光岩,彭歆. 腮腺微小肿瘤的临床病理研究[J]. 北京大学学报(医学版), 2022, 54(2): 335-339. |
| [15] | 薛江,张建运,时瑞瑞,谢晓艳,白嘉英,李铁军. 105例口腔颅颌面部纤维性结构不良的临床病理分析[J]. 北京大学学报(医学版), 2022, 54(1): 54-61. |
|
||