北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (6): 1088-1092. doi: 10.19723/j.issn.1671-167X.2020.06.016
Ro52抗体与其他肌炎抗体共阳性的相关性研究
- 北京大学第一医院神经内科,北京 100034
Correlation study on anti-Ro52 antibodies frequently co-occur with other myositis-specific and myositis-associated autoantibodies
Yi-ming ZHENG,Hong-jun HAO,Yi-lin LIU,Jing GUO,Ya-wen ZHAO,Wei ZHANG,Yun YUAN( )
)
- Department of Neurology, Peking University First Hospital, Beijing 100034, China
摘要:
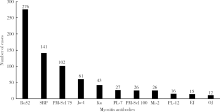
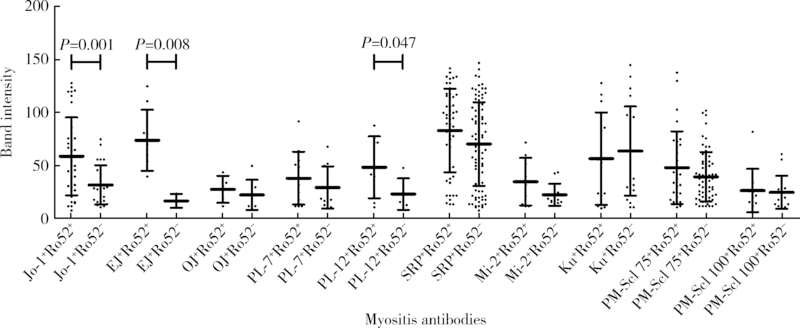
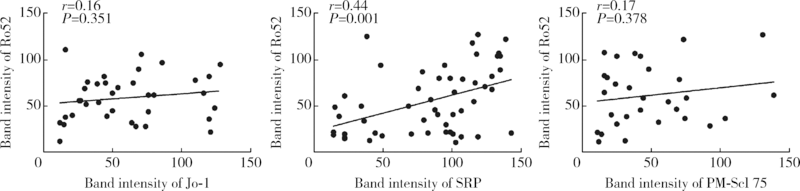
目的:观察Ro52抗体与其他肌炎抗体共阳性的相关规律。方法:回顾性分析2010—2016年在北京大学第一医院应用线性免疫印迹法检测的1 509例临床疑诊为炎症性肌病患者血清中11种肌炎特异性或相关性抗体(Jo-1、PL-7、PL-12、EJ、OJ、Mi-2、SRP、Ku、PM-Scl 75、PM-Scl 100、Ro52)的检查结果,分析Ro52抗体与其他肌炎抗体共阳性的相关规律,用SPSS 17.0以及Graph Pad PRISM软件进行统计学分析及作图。结果:Ro52抗体阳性率达18.3%(276/1 509例),为最常检测出的肌炎抗体。Ro52抗体阳性的患者中有51.8%合并其他肌炎抗体,合并SRP抗体的比例最高(18.8%),其次为Jo-1抗体(13.0%)。除OJ抗体外,其他肌炎抗体阳性患者最常合并的另一种抗体均为Ro52,其共阳性率在PM-Scl 75阳性组最低(30.4%)、在EJ抗体阳性组最高(80.0%)。抗合成酶抗体阳性的患者有57.3%合并Ro52抗体,显著高于非抗合成酶抗体阳性的患者(35.2%, χ2=18.916,P<0.001)。Jo-1抗体、EJ抗体以及SRP抗体阳性的患者中,Ro52抗体共阳性组的抗体谱带强度均显著高于相应的Ro52抗体阴性组(P<0.05)。SRP抗体谱带强度与Ro52抗体谱带强度呈显著正相关(r=0.44,P=0.001)。结论:Ro52抗体是其他肌炎抗体阳性患者常合并出现的一种抗体,尤其是抗合成酶抗体阳性的患者,是否合并出现Ro52抗体可能与该肌炎抗体的滴度相关。
中图分类号:
- R593.2
| [1] |
Cruellas MG, Viana VS, Levy-Neto M, et al. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis[J]. Clinics (Sao Paulo), 2013,68(7):909-914.
doi: 10.6061/clinics |
| [2] |
Lee A. A review of the role and clinical utility of anti-Ro52/TRIM21 in systemic autoimmunity[J]. Rheumatol Int, 2017,37(8):1323-1333.
doi: 10.1007/s00296-017-3718-1 pmid: 28417151 |
| [3] |
Rutjes SA, Vree EW, Jongen P, et al. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy[J]. Clin Exp Immunol, 1997,109(1):32-40.
doi: 10.1046/j.1365-2249.1997.4081308.x pmid: 9218821 |
| [4] |
Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: A critical review[J]. J Autoimmun, 2012,39(1/2):77-82.
doi: 10.1016/j.jaut.2012.01.014 |
| [5] |
Damoiseaux J, Boesten K, Giesen J, et al. Evaluation of a novel line-blot immunoassay for the detection of antibodies to extractable nuclear antigens[J]. Ann N Y Acad Sci, 2005,1050:340-347.
doi: 10.1196/annals.1313.036 pmid: 16014550 |
| [6] |
Frank MB, Mccubbin V, Trieu E, et al. The association of anti-Ro52 autoantibodies with myositis and scleroderma autoantibodies[J]. J Autoimmun, 1999,12(2):137-142.
doi: 10.1006/jaut.1998.0265 pmid: 10047434 |
| [7] |
Brouwer R, Hengstman GJ, Vree EW, et al. Autoantibody profiles in the sera of European patients with myositis[J]. Ann Rheum Dis, 2001,60(2):116-123.
doi: 10.1136/ard.60.2.116 pmid: 11156543 |
| [8] |
Venables PJ. Antibodies to Jo-1 and Ro-52: why do they go together?[J]. Clin Exp Immunol, 1997,109(3):403-405.
doi: 10.1046/j.1365-2249.1997.4761369.x pmid: 9328112 |
| [9] |
Bundell C, Rojana-Udomsart A, Mastaglia F, et al. Diagnostic performance of a commercial immunoblot assay for myositis antibody testing[J]. Pathology, 2016,48(4):363-366.
doi: 10.1016/j.pathol.2016.03.012 pmid: 27114370 |
| [10] |
Marie I, Hatron PY, Dominique S, et al. Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody[J]. Semin Arthritis Rheum, 2012,41(6):890-899.
doi: 10.1016/j.semarthrit.2011.09.008 pmid: 22078416 |
| [11] | 孟令超, 李毅, 张巍, 等. Jo-1综合征的临床及骨骼肌病理特点[J]. 中华医学杂志, 2016,96(29):2352-2355. |
| [12] |
Yamasaki Y, Satoh M, Mizushima M, et al. Clinical subsets associated with different anti-aminoacyl transfer RNA synthetase antibodies and their association with coexisting anti-Ro52[J]. Mod Rheumatol, 2016,26(3):403-409.
doi: 10.3109/14397595.2015.1091155 pmid: 26344678 |
| [13] |
Bauhammer J, Blank N, Max R, et al. Rituximab in the treatment of Jo1 antibody-associated antisynthetase syndrome: Anti-Ro52 positivity as a marker for severity and treatment response[J]. J Rheumatol, 2016,43(8):1566-1574.
doi: 10.3899/jrheum.150844 pmid: 27252419 |
| [14] |
Ben-Chetrit E, Chan EK, Sullivan KF, et al. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle[J]. J Exp Med, 1988,167(5):1560-1571.
doi: 10.1084/jem.167.5.1560 pmid: 3367095 |
| [15] |
Peene I, Meheus L, de Keyser S, et al. Anti-Ro52 reactivity is an independent and additional serum marker in connective tissue disease[J]. Ann Rheum Dis, 2002,61(10):929-933.
doi: 10.1136/ard.61.10.929 pmid: 12228166 |
| [16] |
Menendez A, Gomez J, Escanlar E, et al. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection[J]. Autoimmunity, 2013,46(1):32-39.
pmid: 23039326 |
| [17] |
Srivastava P, Dwivedi S, Misra R. Myositis-specific and myositis-associated autoantibodies in Indian patients with inflammatory myositis[J]. Rheumatol Int, 2016,36(7):935-943.
doi: 10.1007/s00296-016-3494-3 pmid: 27193471 |
| [18] |
Gunnarsson R, El-Hage F, Aalokken TM, et al. Associations between anti-Ro52 antibodies and lung fibrosis in mixed connective tissue disease[J]. Rheumatology (Oxford), 2016,55(1):103-108.
doi: 10.1093/rheumatology/kev300 |
| [1] | 李正芳,罗采南,武丽君,吴雪,孟新艳,陈晓梅,石亚妹,钟岩. 抗氨基甲酰化蛋白抗体在诊断类风湿关节炎中的应用价值[J]. 北京大学学报(医学版), 2024, 56(4): 729-734. |
| [2] | 赖展鸿,李嘉辰,贠泽霖,张永刚,张昊,邢晓燕,邵苗,金月波,王乃迪,李依敏,李玉慧,栗占国. 特发性炎性肌病完全临床应答相关因素的单中心真实世界研究[J]. 北京大学学报(医学版), 2024, 56(2): 284-292. |
| [3] | 孟彦宏,陈怡帆,周培茹. CENP-B抗体阳性的原发性干燥综合征患者的临床和免疫学特征[J]. 北京大学学报(医学版), 2023, 55(6): 1088-1096. |
| [4] | 扶琼,叶霜. 嵌合抗原受体T细胞治疗在自身免疫疾病中的应用和思考[J]. 北京大学学报(医学版), 2023, 55(6): 953-957. |
| [5] | 张璐,陈澄,翁梅婷,郑爱萍,苏美玲,王庆文,蔡月明. 狼疮肾炎患者肾小管间质损伤的自身抗体特征[J]. 北京大学学报(医学版), 2022, 54(6): 1094-1098. |
| [6] | 张琳崎,赵静,王红彦,王宗沂,李英妮,汤稷旸,李思莹,曲进锋,赵明威. 抗ENO1抗体与狼疮性视网膜病变的相关性[J]. 北京大学学报(医学版), 2022, 54(6): 1099-1105. |
| [7] | 邢晓燕,张筠肖,朱冯赟智,王一帆,周新尧,李玉慧. 皮肌炎合并巨噬细胞活化综合征5例[J]. 北京大学学报(医学版), 2022, 54(6): 1214-1218. |
| [8] | 应沂岑,唐琦,杨恺惟,米悦,范宇,虞巍,宋毅,何志嵩,周利群,李学松. 泌尿肿瘤免疫检查点抑制剂相关性肌炎的临床特征[J]. 北京大学学报(医学版), 2022, 54(4): 644-651. |
| [9] | 张朴丽,杨红霞,张立宁,葛勇鹏,彭清林,王国春,卢昕. 血清YKL-40在诊断抗黑色素瘤分化相关基因5阳性皮肌炎合并严重肺损伤中的价值[J]. 北京大学学报(医学版), 2021, 53(6): 1055-1060. |
| [10] | 罗澜,邢晓燕,肖云抒,陈珂彦,朱冯赟智,张学武,李玉慧. 抗合成酶综合征合并心脏受累患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1078-1082. |
| [11] | 肖云抒,朱冯赟智,罗澜,邢晓燕,李玉慧,张学武,沈丹华. 88例重叠肌炎的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1088-1093. |
| [12] | 伊文霞,魏翠洁,吴晔,包新华,熊晖,常杏芝. 长疗程利妥昔单抗治疗难治性幼年型特发性炎症性肌病3例[J]. 北京大学学报(医学版), 2021, 53(6): 1191-1195. |
| [13] | 吴燕芳,高飞,林滇恬,陈志涵,林禾. 托法替布联合治疗抗MDA5抗体阳性的无肌病皮肌炎并发快速进展型间质性肺病5例临床分析[J]. 北京大学学报(医学版), 2021, 53(5): 1012-1016. |
| [14] | 甘雨舟,李玉慧,张丽华,马琳,何文雯,金月波,安媛,栗占国,叶华. 临床无肌病性皮肌炎与皮肌炎临床及免疫学特征比较[J]. 北京大学学报(医学版), 2020, 52(6): 1001-1008. |
| [15] | 赵静,孙峰,李云,赵晓珍,徐丹,李英妮,李玉慧,孙晓麟. 抗α-1C微管蛋白抗体在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2020, 52(6): 1009-1013. |
|
||