北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (3): 613-622. doi: 10.19723/j.issn.1671-167X.2021.03.030
肾部分切除术前CT三维可视化评估标准的初步探究
李新飞1,*,彭意吉1,*,余霄腾1,*,熊盛炜1,程嗣达1,丁光璞1,杨昆霖1,唐琦1,Δ( ),米悦1,吴静云1,张鹏2,谢家馨1,郝瀚1,王鹤3,邱建星3,杨建4,李学松1,Δ(
),米悦1,吴静云1,张鹏2,谢家馨1,郝瀚1,王鹤3,邱建星3,杨建4,李学松1,Δ( ),周利群1
),周利群1
- 1.北京大学第一医院泌尿外科,北京大学泌尿外科研究所,国家泌尿、男性生殖系肿瘤研究中心,北京 100034
2.应急总医院泌尿外科,北京 100028
3.北京大学第一医院影像科,北京 100034
4.北京理工大学光电学院,北京市混合现实与新型显示工程技术研究中心,北京 100081
Three dimensional nephrometry system for partial nephrectomy: Our initial exploration
LI Xin-fei1,*,PENG Yi-ji1,*,YU Xiao-teng1,*,XIONG Sheng-wei1,CHENG Si-da1,DING Guang-pu1,YANG Kun-lin1,TANG Qi1,Δ( ),MI Yue1,WU Jing-yun1,ZHANG Peng2,XIE Jia-xin1,HAO Han1,WANG He3,QIU Jian-xing3,YANG Jian4,LI Xue-song1,Δ(
),MI Yue1,WU Jing-yun1,ZHANG Peng2,XIE Jia-xin1,HAO Han1,WANG He3,QIU Jian-xing3,YANG Jian4,LI Xue-song1,Δ( ),ZHOU Li-qun1
),ZHOU Li-qun1
- 1. Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Cancer Center, Beijing 100034, China
2. Department of Urology, Emergency General Hospital, Beijing 100028, China
3. Department of Radiology, Peking University First Hospital, Beijing 100034, China
4. Beijing Engineering Research Center for Mixed Reality and Advanced Display Technology, School of Optics and Photonics, Beijing Institute of Technology, Beijing 100081, China
摘要:
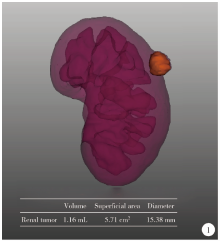
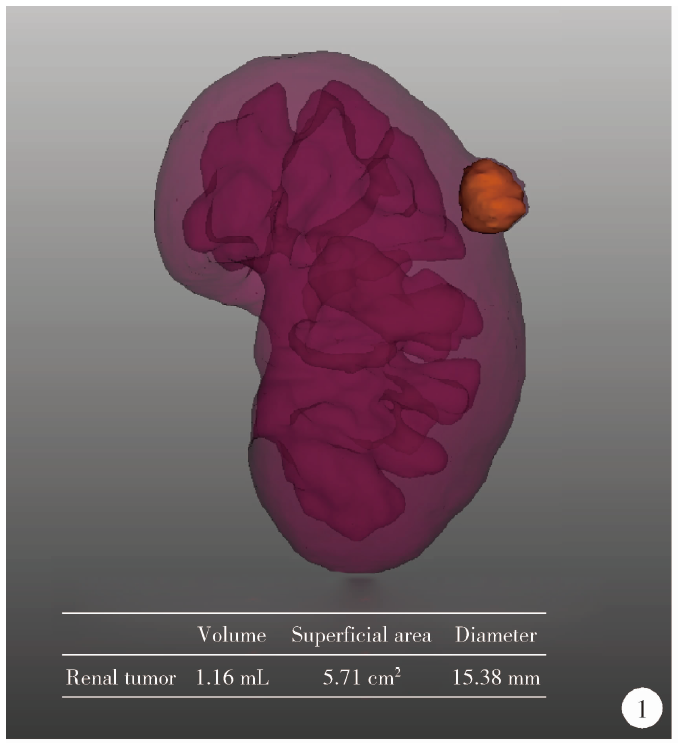
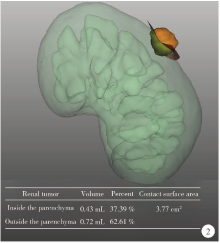
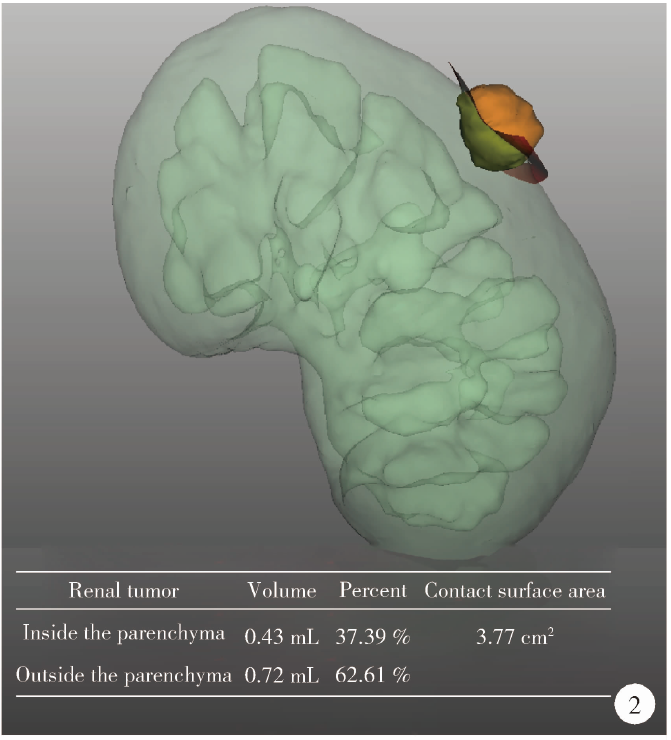
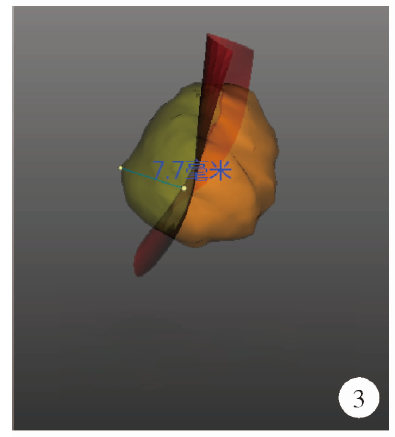
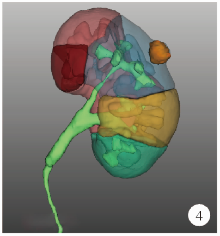
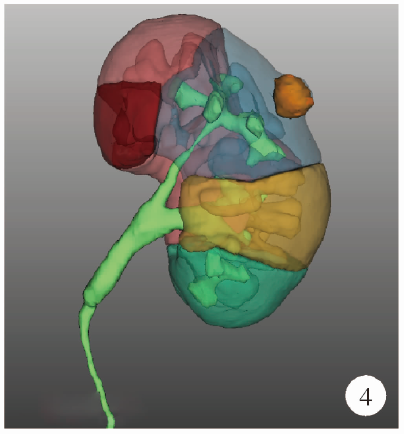
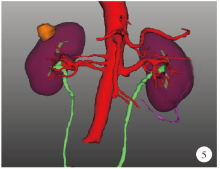
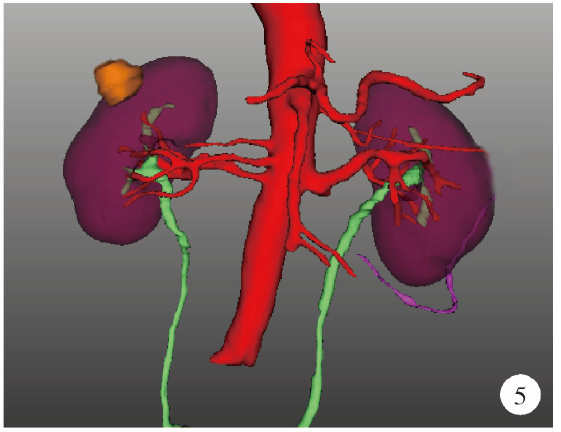
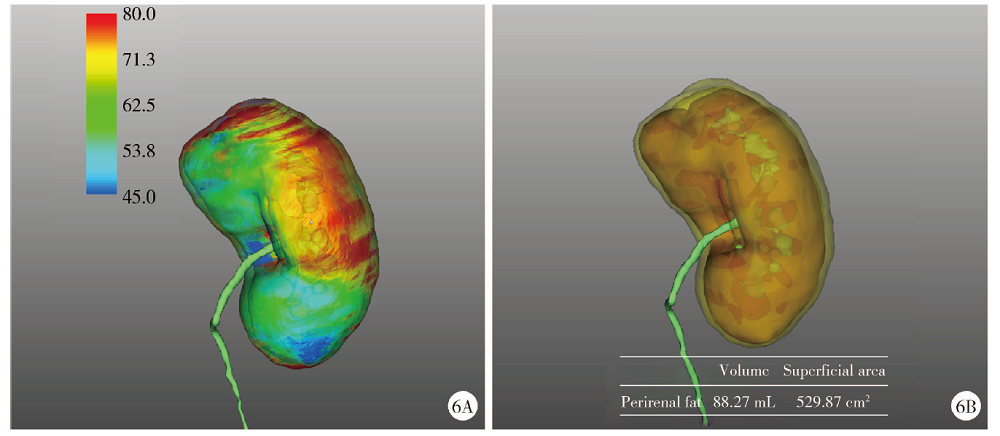
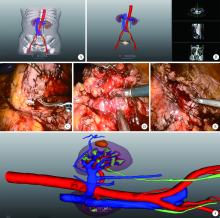
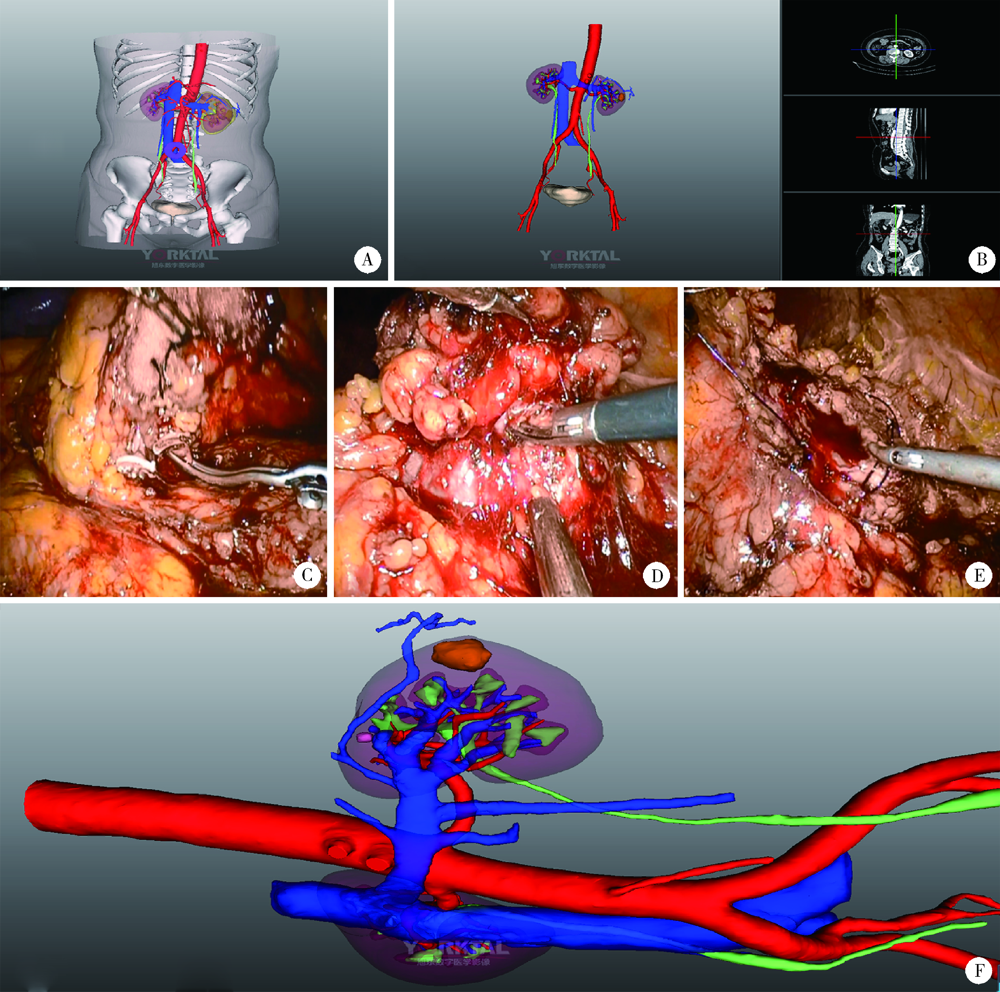
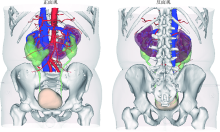
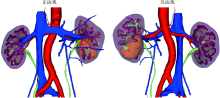
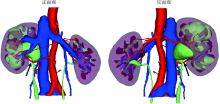
目的: 探索并构建肾肿瘤行肾部分切除术的CT三维可视化术前评估系统及其应用价值。方法: 回顾性收集北京大学第一医院泌尿外科因肾肿瘤行肾部分切除术患者的临床资料做初步探究,同时收集我国16家临床中心因肾肿瘤行肾部分切除术患者的同质化标准数据,应用CT三维可视化系统(IPS系统,Yorktal)评估肿瘤解剖结构、血供等信息,通过归纳和总结构建评估系统,完成虚拟手术设计及术中辅助导航,指导临床手术。结果: 基于泌尿系增强CT建立三维可视化图像,评分系统纳入肿瘤最长径和体积、肿瘤侵入实质内体积占比、肿瘤侵入实质最大深度、肿瘤与肾实质接触面积、肿瘤肾实质接触面平整度、肿瘤所在肾脏分段位置、肾血管变异情况及肾周脂肪。肿瘤平均二维直径为(2.78±1.43) cm,平均三维最大径为(3.09±1.35) cm,术后病理平均大小(3.01±1.38) cm。三维重建肿瘤最大径与术中肾动脉阻断时间延长、术中出血量显著相关(r=0.502,P=0.020;r=0.403,P=0.046)。三维重建及病理肿瘤体积分别为(25.7±48.4) cm3、(33.0±36.4) cm3(P=0.229),三维重建肿瘤体积与术中出血量显著相关(r=0.660,P<0.001),肿瘤侵入肾实质内体积占比与术中肾动脉阻断时间延长、术后并发症的发生显著相关(r=0.410,P=0.041;r=0.587,P=0.005)。肿瘤与肾实质接触面积及是否存在血管变异与围手术期指标及术后并发症未见相关性。完成术前评估的同时,重建后的三维影像可在Touch Viewer系统上进行缩放、旋转、组合显示、颜色调整、透明化、长度体积自动测量及模拟裁切等操作,满足术前虚拟手术规划及术中辅助导航的要求。结论: 三维图像可提供更加直观的解剖结构,清晰显示肿瘤解剖参数及血供、脂肪等信息,CT三维重建肾肿瘤评价系统可帮助预测肾部分切除术手术难度、围术期并发症等。重建的三维可视化图像导入指定程序或机器人操作系统即可完成虚拟手术及术中辅助导航,帮助手术医师更好地把握手术过程。评分系统所包含的指标及各项指标的分值权重需要通过多中心大样本的研究来证实及完善。
中图分类号:
- R737.11
| [1] |
Rossi SH, Klatte T, Usher-Smith J, et al. Epidemiology and screening for renal cancer[J]. World J Urol, 2018,36(9):1341-1353.
doi: 10.1007/s00345-018-2286-7 |
| [2] |
Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2. NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2017,15(6):804-834.
doi: 10.6004/jnccn.2017.0100 |
| [3] |
Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline[J]. J Urol, 2017,198(3):520-529.
doi: 10.1016/j.juro.2017.04.100 |
| [4] |
Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery[J]. Eur Urol, 2009,56(5):786-793.
doi: 10.1016/j.eururo.2009.07.040 |
| [5] |
Kutikov A, Uzzo RG. The R. E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth[J]. J Urol, 2009,182(3):844-853.
doi: 10.1016/j.juro.2009.05.035 |
| [6] |
Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method[J]. J Urol, 2010,183(5):1708-1713.
doi: 10.1016/j.juro.2010.01.005 |
| [7] |
Hew MN, Baseskioglu B, Barwari K, et al. Critical appraisal of the PADUA classification and assessment of the R.E.N.A.L. nephrometry score in patients undergoing partial nephrectomy[J]. J Urol, 2011,186(1):42-46.
doi: 10.1016/j.juro.2011.03.020 |
| [8] | Wadle J, Hetjens S, Winter J, et al. Nephrometry scores: The effect of imaging on routine read-out and prediction of outcome of nephron-sparing surgery[J]. Anticancer Res, 2018,38(5):3037-3041. |
| [9] | Porpiglia F, Amparore D, Checcucci E, et al. Current use of three-dimensional model technology in urology: A road map for personalised surgical planning[J]. Eur Urol Focus, 2018,4(5):652-656. |
| [10] |
Cartiaux O, Paul L, Francq BG, et al. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery[J]. Ann Biomed Eng, 2014,42(1):205-213.
doi: 10.1007/s10439-013-0890-7 pmid: 23963884 |
| [11] |
Wu J, Li Y, Zhang Y. Use of intraoral scanning and 3-dimensional printing in the fabrication of a removable partial denture for a patient with limited mouth opening[J]. J Am Dent Assoc, 2017,148(5):338-341.
doi: 10.1016/j.adaj.2017.01.022 |
| [12] |
Porpiglia F, Amparore D, Checcucci E, et al. Three-dimensional virtual imaging of renal tumours: A new tool to improve the accuracy of nephrometry scores[J]. BJU Int, 2019,124(6):945-954.
doi: 10.1111/bju.14894 |
| [13] |
Tannus M, Goldman SM, Andreoni C. Practical and intuitive surgical approach renal ranking to predict outcomes in the management of renal tumors: A novel score tool[J]. J Endourol, 2014,28(4):487-492.
doi: 10.1089/end.2013.0148 |
| [14] | Nisen H, Ruutu M, Glucker E, et al. Renal tumour invasion index as a novel anatomical classification predicting urological complications after partial nephrectomy[J]. Scand J Urol, 2014,48(1):41-51. |
| [15] |
Leslie S, Gill IS, de Castro AA, et al. Renal tumor contact surface area: A novel parameter for predicting complexity and outcomes of partial nephrectomy[J]. Eur Urol, 2014,66(5):884-893.
doi: 10.1016/j.eururo.2014.03.010 |
| [16] |
Hsieh PF, Wang YD, Huang CP, et al. A mathematical method to calculate tumor contact surface area: An effective parameter to predict renal function after partial nephrectomy[J]. J Urol, 2016,196(1):33-40.
doi: 10.1016/j.juro.2016.01.092 |
| [17] |
Takagi T, Yoshida K, Kondo T, et al. Association between tumor contact surface area and parenchymal volume change in robot-assisted laparoscopic partial nephrectomy carried out using the enucleation technique[J]. Int J Urol, 2019,26(7):745-751.
doi: 10.1111/iju.2019.26.issue-7 |
| [18] |
Kiziloz H, Dorin R, Finnegan KT, et al. The impact of body mass index on perioperative outcomes in robot-assisted laparoscopic partial nephrectomy[J]. J Endourol, 2013,27(8):1000-1007.
doi: 10.1089/end.2012.0665 |
| [19] |
Gong EM, Orvieto MA, Lyon MB, et al. Analysis of impact of body mass index on outcomes of laparoscopic renal surgery[J]. Urology, 2007,69(1):38-43.
doi: 10.1016/j.urology.2006.09.020 |
| [20] |
Macleod LC, Hsi RS, Gore JL, et al. Perinephric fat thickness is an independent predictor of operative complexity during robot-assisted partial nephrectomy[J]. J Endourol, 2014,28(5):587-591.
doi: 10.1089/end.2013.0647 |
| [1] | 杨捷,冯杰莉,张树栋,马潞林,郑清. 经食管超声心动图在肾切除术联合Mayo Ⅲ~Ⅳ级静脉瘤栓取栓术不同手术方式中的临床作用[J]. 北京大学学报(医学版), 2024, 56(4): 631-635. |
| [2] | 王滨帅,邱敏,张前进,田茂锋,刘磊,王国良,陆敏,田晓军,张树栋. 6例肾尤文肉瘤伴静脉瘤栓的诊治[J]. 北京大学学报(医学版), 2024, 56(4): 636-639. |
| [3] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [4] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [5] | 邱敏,宗有龙,王滨帅,杨斌,徐楚潇,孙争辉,陆敏,赵磊,卢剑,刘承,田晓军,马潞林. 腹腔镜肾部分切除术治疗中高复杂程度肾肿瘤的效果[J]. 北京大学学报(医学版), 2023, 55(5): 833-837. |
| [6] | 张铨,宋海峰,马冰磊,张喆楠,周朝晖,李傲林,刘军,梁磊,朱时雨,张骞. 术前预后营养指数可作为预测非转移性肾细胞癌预后的指标[J]. 北京大学学报(医学版), 2023, 55(1): 149-155. |
| [7] | 周利群,徐纯如. 机器人时代中央型肾肿瘤的手术治疗策略[J]. 北京大学学报(医学版), 2022, 54(4): 587-591. |
| [8] | 韩松辰,黄子雄,刘慧鑫,徐涛. 单侧肾细胞癌根治性切除术后的肾功能代偿[J]. 北京大学学报(医学版), 2021, 53(4): 680-685. |
| [9] | 邱敏,费月阳,邓绍晖,刘承,卢剑,何为,陆敏,田晓军,张树栋,马潞林. 后肾腺瘤的诊治经验及文献回顾[J]. 北京大学学报(医学版), 2021, 53(2): 417-419. |
| [10] | 毕海,黄毅,马潞林,陆敏,张树栋,张洪宪. 3例肾尤文肉瘤合并下腔静脉癌栓的诊治[J]. 北京大学学报(医学版), 2020, 52(5): 985-989. |
| [11] | 纪翔,王义,周哲,赵子臣,果宏峰,王刚,张志宏,晋连超,孙国锋,赵文锋,汪磊,贺利军,李宁忱,那彦群. 后腹腔镜超声引导下微波消融治疗复杂肾肿瘤[J]. 北京大学学报(医学版), 2020, 52(4): 785-789. |
| [12] | 田晓军,邱敏,刘茁,肖若陶,黄毅,王国良,侯小飞,张树栋,庄申榕,马潞林. 微创手术治疗肾癌合并Mayo 0~2级静脉癌栓的单中心研究[J]. 北京大学学报(医学版), 2018, 50(6): 1053-1056. |
| [13] | 徐奔,张喆楠,罗程,宋海峰,张骞. 后腹腔镜下肿瘤吸除术与肾部分切除术治疗肾血管平滑肌脂肪瘤的安全性与有效性对比[J]. 北京大学学报(医学版), 2018, 50(4): 700-704. |
| [14] | 黄子雄,张晓鹏,董森,刘士军,杨荣利,周宇石,马伟国. 肾黏液性小管和梭形细胞癌合并骨转移1例及文献回顾[J]. 北京大学学报(医学版), 2018, 50(4): 732-735. |
| [15] | 马伟国,秦彩朋,于路平,张晓鹏,刘士军,王永顺, 白文俊,徐涛. 后腹腔镜下孤立肾采用肾动脉非阻断法行保留肾单位肾肿瘤剜除术的疗效评价[J]. 北京大学学报(医学版), 2018, 50(4): 759-761. |
|
||