北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (4): 730-737. doi: 10.19723/j.issn.1671-167X.2020.04.026
能谱CT诊断非小细胞肺癌纵隔淋巴结转移的应用价值
- 北京大学第三医院放射科,北京 100191
Comparative imaging study of mediastinal lymph node from pre-surgery dual energy CT versus post-surgeron verifications in non-small cell lung cancer patients
Qiao ZHU,Cui REN,Yan ZHANG,Mei-jiao LI,Xiao-hua WANG( )
)
- Department of Radiology, Peking University Third Hospital, Beijing 100191, China
摘要:
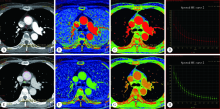
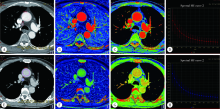
目的: 探讨能谱CT (dual energy CT, DECT) 诊断非小细胞肺癌 (non-small cell lung cancer, NSCLC) 纵隔淋巴结转移的应用价值。方法: 选择2018年4月至2019年10月在北京大学第三医院接受胸部DECT检查且经术后病理诊断证实的NSCLC患者病例资料进行回顾性分析,共收集到病例57 例,两名放射科医师共同分析患者术前CT图像,将轴位图像上所有短径 (short-axis diameter, S)≥5 mm的纵隔淋巴结纳入本研究。测量淋巴结形态学参数长径(long-axis diameter, L)、S、短径与长径比值(ratio of short-axis diameter to long-axis diameter, S/L)以及能谱参数动脉期及静脉期碘浓度 (iodine concentration, IC)、标准化碘浓度 (normalized iodine concentration, NIC)、能谱曲线斜率及有效原子序数。比较转移与非转移淋巴结形态学指标及其能谱参数的差异,将有统计学差异的参数纳入Logistic回归方程筛选出有诊断价值的参数,并生成诊断淋巴结转移的联合变量,对淋巴结S、静脉期NIC及联合变量进行受试者工作特征 (receiver operating characteristic, ROC)曲线分析。结果: 57例患者中,术后病理诊断证实转移淋巴结49 枚,非转移淋巴结938 枚。CT轴位上共检出S≥5 mm纵隔淋巴结163 枚 (转移淋巴结49 枚,非转移淋巴结114 枚)。转移淋巴结的S、L及S/L均显著大于非转移淋巴结 (P<0.05), 转移淋巴结的能谱参数均显著低于非转移性淋巴结 (P<0.05)。S是诊断淋巴结转移的最佳单一形态学指标,ROC曲线下面积 (area under curve, AUC) 为0.752,阈值8.5 mm,灵敏度67.4%,特异度73.7%,准确率71.8%。静脉期NIC为最佳单一能谱参数,AUC为0.861,阈值0.53,灵敏度95.9%,特异度70.2%,准确率77.9%。多因素分析显示S、静脉期NIC是转移淋巴结的独立预测因子。联合S、静脉期NIC诊断淋巴结转移的AUC为0.895,灵敏度 79.6%,特异度 87.7%,准确率85.3%,明显高于S (P<0.001)、静脉期NIC (P=0.037)。结论: DECT定量参数鉴别NSCLC患者纵隔淋巴结转移的价值优于形态学参数,联合S和静脉期NIC可提高术前诊断淋巴结转移的准确率。
中图分类号:
- R734.2
| [1] | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018,68(1):7-30. |
| [2] |
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods[J]. Int J Cancer, 2019,144(8):1941-1953.
doi: 10.1002/ijc.31937 pmid: 30350310 |
| [3] | 中华医学会, 中华医学会肿瘤学分会, 中华医学会杂志社. 中华医学会肺癌临床诊疗指南(2018版)[J]. 中华肿瘤杂志, 2018,40(12):935-964. |
| [4] |
Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging[J]. J Nucl Med, 2004,45(9):1509-1518.
pmid: 15347718 |
| [5] | Vansteenkiste J, Dooms C, De Leyn P. Early stage non-small cell lung cancer: challenges in staging and adjuvant treatment: evidence-based staging[J]. Ann Oncol, 2010, 21(Suppl 7): vii189-vii195. |
| [6] | 宁先英, 李浩, 杨明, 等. CT能谱定量分析对肺腺癌与鳞癌的鉴别诊断价值[J]. 放射学实践, 2017,32(3):237-241. |
| [7] |
Lv P, Lin XZ, Li J, et al. Differentiation of small hepatic hemangioma from small hepatocellular carcinoma: recently introduced spectral CT method[J]. Radiology, 2011,259(3):720-729.
doi: 10.1148/radiol.11101425 pmid: 21357524 |
| [8] |
Martin SS, Weidinger S, Czwikla R, et al. Iodine and fat quantification for differentiation of adrenal gland adenomas from metas-tases using third-generation dual-source dual-energy computed tomography[J]. Invest Radiol, 2018,53(3):173-178.
pmid: 28990974 |
| [9] |
Muenzel D, Lo GC, Yu HS, et al. Material density iodine images in dual-energy CT: detection and characterization of hypervascular liver lesions compared to magnetic resonance imaging[J]. Eur J Radiol, 2017,95(10):300-306.
doi: 10.1016/j.ejrad.2017.08.035 |
| [10] |
Liu X, Ouyang D, Li H, et al. Papillary thyroid cancer: dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes[J]. Radiology, 2015,275(1):167-176.
pmid: 25521777 |
| [11] |
De Leyn P, Vansteenkiste J, Cuypers P, et al. Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan[J]. Eur J Cardiothorac Surg, 1997,12(5):706-712.
doi: 10.1016/s1010-7940(97)00253-4 pmid: 9458140 |
| [12] |
Fukuya T, Honda H, Hayashi T, et al. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer[J]. Radiology, 1995,197(3):705-711.
pmid: 7480743 |
| [13] |
Yoshimura G, Sakurai T, Oura S, et al. Evaluation of axillary lymph node status in breast cancer with MRI[J]. Breast Cancer, 1999,6(3):249-258.
pmid: 11091725 |
| [14] |
Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: an initial experience[J]. Eur J Radiol, 2016,85(6):1219-1223.
doi: 10.1016/j.ejrad.2016.03.030 pmid: 27161073 |
| [15] |
Rizzo S, Radice D, Femia M, et al. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT[J]. Eur Radiol, 2018,28(2):760-769.
doi: 10.1007/s00330-017-5015-5 pmid: 28835993 |
| [16] | Yang Z, Zhang X, Fang M, et al. Preoperative diagnosis of regional lymph node metastasis of colorectal cancer with quantitative parameters from dual-energy CT[J]. AJR Am J Roentgenol, 2019,213(6):1-9. |
| [17] |
Zhang X, Zheng C, Yang Z, et al. Axillary sentinel lymph nodes in breast cancer: quantitative evaluation at dual-energy CT[J]. Radiology, 2018,289(2):337-346.
doi: 10.1148/radiol.2018180544 pmid: 30152748 |
| [18] | Yang F, Dong J, Wang X, et al. Non-small cell lung cancer: spectral computed tomography quantitative parameters for preoperative diagnosis of metastatic lymph nodes[J]. Eur J Radiol, 2017,89(4):129-135. |
| [19] |
Lin LY, Zhang Y, Suo ST, et al. Correlation between dual-energy spectral CT imaging parameters and pathological grades of non-small cell lung cancer[J]. Clin Radiol, 2018, 73(4): 412.e1-412.e7.
doi: 10.1016/j.crad.2017.10.020 pmid: 29195660 |
| [20] | 崔元龙, 许毛荣, 文智. 能谱CT定量参数对非小细胞肺癌纵隔淋巴结转移中的应用价值[J]. 临床放射学杂志, 2019,38(5):825-829. |
| [21] | 叶亚君, 莫淑琼. CT能谱成像鉴别诊断纵隔淋巴结良恶性的临床意义[J]. 现代医用影像学, 2018,27(8):2702-2703. |
| [22] |
Pan Z, Pang L, Ding B, et al. Gastric cancer staging with dual energy spectral CT imaging[J]. PLoS One, 2013,8(2):e53651.
doi: 10.1371/journal.pone.0053651 pmid: 23424614 |
| [23] | Yang L, Luo D, Li L, et al. Differentiation of malignant cervical lymphadenopathy by dual-energy CT: a preliminary analysis[J]. Sci Rep, 2016,6(1):31020. |
| [1] | 钟华, 李原, 徐丽玲, 白明欣, 苏茵. 18F-FDG PET/CT在风湿免疫病中的应用[J]. 北京大学学报(医学版), 2024, 56(5): 853-859. |
| [2] | 凌晓彤,屈留洋,郑丹妮,杨静,闫雪冰,柳登高,高岩. 牙源性钙化囊肿与牙源性钙化上皮瘤的三维影像特点[J]. 北京大学学报(医学版), 2024, 56(1): 131-137. |
| [3] | 段登辉,WANGHom-Lay,王恩博. 可吸收胶原膜在颊侧袋形瓣引导性骨再生手术中的作用: 一项回顾性影像学队列研究[J]. 北京大学学报(医学版), 2023, 55(6): 1097-1104. |
| [4] | 李东,邸吉廷,熊焰. 程序性细胞死亡1-配体1在不同免疫组织化学染色方法的一致性比较[J]. 北京大学学报(医学版), 2023, 55(2): 339-342. |
| [5] | 付玉,胡鑫浓,崔圣洁,施捷. 骨性Ⅱ类高角错 |
| [6] | 王书磊,高阳旭,张宏武,杨海波,李辉,李宇,沈笠雪,姚红新. 儿童基底节区生殖细胞瘤30例临床分析[J]. 北京大学学报(医学版), 2022, 54(2): 222-226. |
| [7] | 杨刚,胡文杰,曹洁,柳登高. 牙周健康的上颌前牙唇侧嵴顶上牙龈的三维形态分析[J]. 北京大学学报(医学版), 2021, 53(5): 990-994. |
| [8] | 李新飞, 彭意吉, 余霄腾, 熊盛炜, 程嗣达, 丁光璞, 杨昆霖, 唐琦, 米悦, 吴静云, 张鹏, 谢家馨, 郝瀚, 王鹤, 邱建星, 杨建, 李学松, 周利群. 肾部分切除术前CT三维可视化评估标准的初步探究[J]. 北京大学学报(医学版), 2021, 53(3): 613-622. |
| [9] | 周境,刘怡. 不同垂直骨面型骨性Ⅱ类青少年女性颞下颌关节锥形束CT测量分析[J]. 北京大学学报(医学版), 2021, 53(1): 109-119. |
| [10] | 高璐,谷岩. 中国人群腭中缝形态特点分期与Demirjian牙龄的相关性[J]. 北京大学学报(医学版), 2021, 53(1): 133-138. |
| [11] | 袁源,郎宁,袁慧书. CT能谱曲线在脊柱转移瘤和感染性病变中的鉴别诊断价值[J]. 北京大学学报(医学版), 2021, 53(1): 183-187. |
| [12] | 李蓬,朴牧子,胡洪成,王勇,赵一姣,申晓婧. 经嵴顶上颌窦底提升术后不植骨同期种植的影像研究[J]. 北京大学学报(医学版), 2021, 53(1): 95-101. |
| [13] | 欧阳雨晴,倪莲芳,刘新民. 恶性孤立性肺结节患者预后因素分析[J]. 北京大学学报(医学版), 2020, 52(1): 158-162. |
| [14] | 李玉冰,孙丽莎,孙志鹏,谢晓艳,张建运,张祖燕,赵燕平,马绪臣. 腮腺CT影像报告与数据系统的初步研究[J]. 北京大学学报(医学版), 2020, 52(1): 83-89. |
| [15] | 张旭初,张建华,王荣福,范岩,付占立,闫平,赵光宇,白艳霞. 18F-FDG PET/CT联合多种肿瘤标志物在结直肠中分化腺癌术后复发及转移中的应用价值[J]. 北京大学学报(医学版), 2019, 51(6): 1071-1077. |
|
||