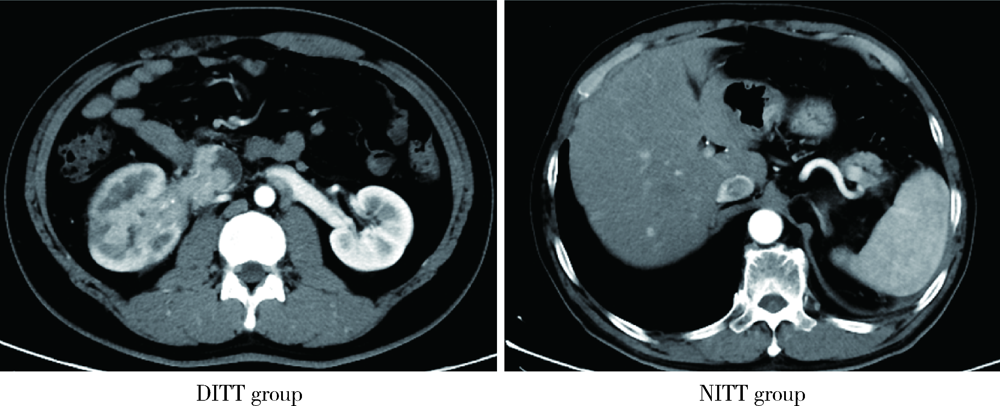
北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (4): 665-670. doi: 10.19723/j.issn.1671-167X.2021.04.007
癌栓粘连血管壁对非转移性肾细胞癌合并下腔静脉癌栓患者手术及预后的影响
赵勋,颜野,黄晓娟,董靖晗,刘茁,张洪宪,刘承( ),马潞林(
),马潞林( )
)
- 北京大学第三医院泌尿外科,北京 100191
Influence of deep invasive tumor thrombus on the surgical treatment and prognosis of patients with non-metastatic renal cell carcinoma complicated with venous tumor thrombus
ZHAO Xun,YAN Ye,HUANG Xiao-juan,DONG Jing-han,LIU Zhuo,ZHANG Hong-xian,LIU Cheng( ),MA Lu-lin(
),MA Lu-lin( )
)
- Department of Urology, Peking University Third Hospital, Beijing 100191, China
摘要:
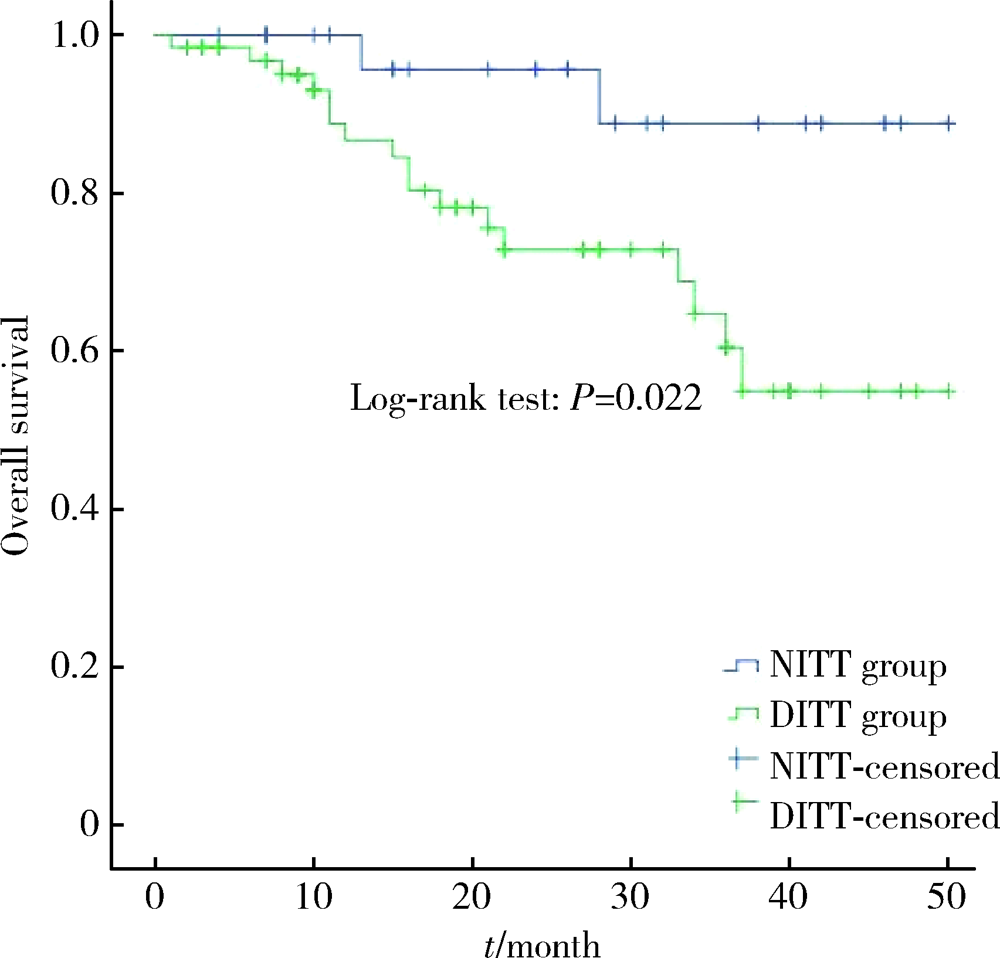
目的: 评估癌栓粘连静脉壁对肾细胞癌伴下腔静脉癌栓患者手术难度和预后的影响。方法: 对北京大学第三医院泌尿外科于2017年1月至2020年6月收治的94例非转移性肾细胞癌合并下腔静脉癌栓患者进行回顾性队列研究,收集患者的一般情况、临床病理特征、手术及生存信息。按术中发现癌栓粘连静脉壁为标准将患者分为两组,其中64例为癌栓粘连静脉壁组(deep invasive tumor thrombus, DITT), 30例为非粘连组(non-invasive tumor thrombus, NITT)。分别采用卡方检验、t检验和Mann-Whitney U检验进行两组间分类变量和连续变量的单因素比较,绘制Kaplan-Meier曲线并进行多变量Cox回归分析以评估癌栓粘连静脉壁对患者预后的影响。结果: 与NITT组相比,DITT组患者的手术难度明显增加,主要表现为手术时间更长(362.5 vs. 307.5 min,P=0.010),手术出血量更多(1 200 vs. 450 mL,P=0.006),围术期输血量更多(800 vs. 0 mL,P=0.021),血浆输注量更多(200 vs. 0 mL,P=0.001),开放手术占比更高(70.3% vs. 36.7%,P=0.002),术后住院时间更长(9.5 vs. 8.0 d,P=0.036),且发生术后并发症的比例更高(46.9% vs. 13.8%,P=0.002)。DITT与患者的总生存期更差呈正相关(P=0.022),即使在多因素分析中,DITT仍是影响肾细胞癌伴下腔静脉癌栓患者术后总生存率的不良预后因素[HR: 4.635 (1.017~21.116),P=0.047]。结论: 对于非转移性肾细胞癌合并下腔静脉癌栓的患者而言,癌栓粘连静脉壁会明显增加其手术难度,并与患者的不良预后相关。
中图分类号:
- R737.11
| [1] |
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018 [J]. Eur J Cancer, 2018, 103:356-387.
doi: S0959-8049(18)30955-9 pmid: 30100160 |
| [2] |
Lardas M, Stewart F, Scrimgeour D, et al. Systematic review of surgical management of nonmetastatic renal cell carcinoma with vena caval thrombus [J]. Eur Urol, 2016, 70(2):265-280.
doi: 10.1016/j.eururo.2015.11.034 |
| [3] |
Quencer KB, Friedman T, Sheth R, et al. Tumor thrombus: incidence, imaging, prognosis and treatment [J]. Cardiovasc Diagn Ther, 2017, 7(Suppl 3):S165-S177.
doi: 10.21037/cdt |
| [4] |
González J, Gorin MA, Garcia-Roig M, et al. Inferior vena cava resection and reconstruction: Technical considerations in the surgical management of renal cell carcinoma with tumor thrombus [J]. Urol Oncol, 2014, 32(1): 34.e19-26.
doi: 10.1016/j.urolonc.2013.01.004 |
| [5] |
Adams LC, Ralla B, Bender YY, et al. Renal cell carcinoma with venous extension: prediction of inferior vena cava wall invasion by MRI [J]. Cancer Imaging, 2018, 18(1):17.
doi: 10.1186/s40644-018-0150-z pmid: 29724245 |
| [6] |
Psutka SP, Boorjian SA, Thompson RH, et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus [J]. BJU Int, 2015, 116(3):388-396.
doi: 10.1111/bju.13005 pmid: 25430786 |
| [7] | Liu Z, Li L, Hong P, et al. A predictive model for tumor invasion of the inferior vena cava wall using multimodal imaging in patients with renal cell carcinoma and inferior vena cava tumor thrombus [J]. Biomed Res Int, 2020, 2020:9530618. |
| [8] |
Li QY, Li N, Huang QB, et al. Contrast-enhanced ultrasound in detecting wall invasion and differentiating bland from tumor thrombus during robot-assisted inferior vena cava thrombectomy for renal cell carcinoma [J]. Cancer Imaging, 2019, 19(1):79.
doi: 10.1186/s40644-019-0265-x |
| [9] |
Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: A population-based study [J]. Lancet Oncol, 2013, 14(2):141-148.
doi: 10.1016/S1470-2045(12)70559-4 |
| [10] |
Xiao R, Xu C, He W, et al. Preoperative anaemia and thrombocytosis predict adverse prognosis in non-metastatic renal cell carcinoma with tumour thrombus [J]. BMC Urol, 2021, 21(1):31.
doi: 10.1186/s12894-021-00796-6 |
| [11] |
Du S, Huang Q, Yu H, et al. Initial series of robotic segmental inferior vena cava resection in left renal cell carcinoma with caval tumor thrombus [J]. Urology, 2020, 142:125-132.
doi: 10.1016/j.urology.2020.03.053 |
| [12] |
Gu L, Li H, Wang Z, et al. A systematic review and meta-analysis of clinicopathologic factors linked to oncologic outcomes for renal cell carcinoma with tumor thrombus treated by radical nephrectomy with thrombectomy [J]. Cancer Treat Rev, 2018, 69:112-120.
doi: 10.1016/j.ctrv.2018.06.014 |
| [13] |
Rodriguez Faba O, Linares E, Tilki D, et al. Impact of microscopic wall invasion of the renal vein or inferior vena cava on cancer-specific survival in patients with renal cell carcinoma and tumor thrombus: A multi-institutional analysis from the International Renal Cell Carcinoma-Venous Thrombus Consortium [J]. Eur Urol Focus, 2018, 4(3):435-441.
doi: S2405-4569(17)30018-4 pmid: 28753848 |
| [14] |
Wang H, Li X, Huang Q, et al. Prognostic role of bland thrombus in patients treated with resection of renal cell carcinoma with infe-rior vena cava tumor thrombus [J]. Urol Oncol, 2021, 39(5): 302.e1-e7.
doi: 10.1016/j.urolonc.2021.02.005 |
| [1] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [2] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [3] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [4] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [5] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [6] | 曾媛媛,谢云,陈道南,王瑞兰. 脓毒症患者发生正常甲状腺性病态综合征的相关因素[J]. 北京大学学报(医学版), 2024, 56(3): 526-532. |
| [7] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [8] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [9] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [10] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [11] | 兰东,刘茁,李宇轩,王国良,田晓军,马潞林,张树栋,张洪宪. 根治性肾切除和静脉癌栓取出术大出血的危险因素[J]. 北京大学学报(医学版), 2023, 55(5): 825-832. |
| [12] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [13] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [14] | 许云屹,苏征征,郑林茂,张孟尼,谭珺娅,杨亚蓝,张梦鑫,徐苗,陈铌,陈雪芹,周桥. 转录通读环状RNA rt-circ-HS促进低氧诱导因子1α表达和肾癌细胞增殖与侵袭[J]. 北京大学学报(医学版), 2023, 55(2): 217-227. |
| [15] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
|
||