北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (3): 572-577. doi: 10.19723/j.issn.1671-167X.2022.03.026
熔融沉积成型3D打印卡托普利与氢氯噻嗪复方片剂的制备与体外评价
- 北京大学药学院药剂学系, 北京大学药学院分子药剂学与新释药系统北京市重点实验室, 北京 100191
Preparation and in vitro evaluation of fused deposition modeling 3D printed compound tablets of captopril and hydrochlorothiazide
Zhi-sheng LI,Hao-nan QIAN,Tian-yuan FAN*( )
)
- Department of Pharmaceutics, Beijing Key Laboratory of Molecular Pharmaceutics and New Drug Delivery Systems, Peking University School of Pharmaceutical Sciences, Beijing 100191, China
摘要:
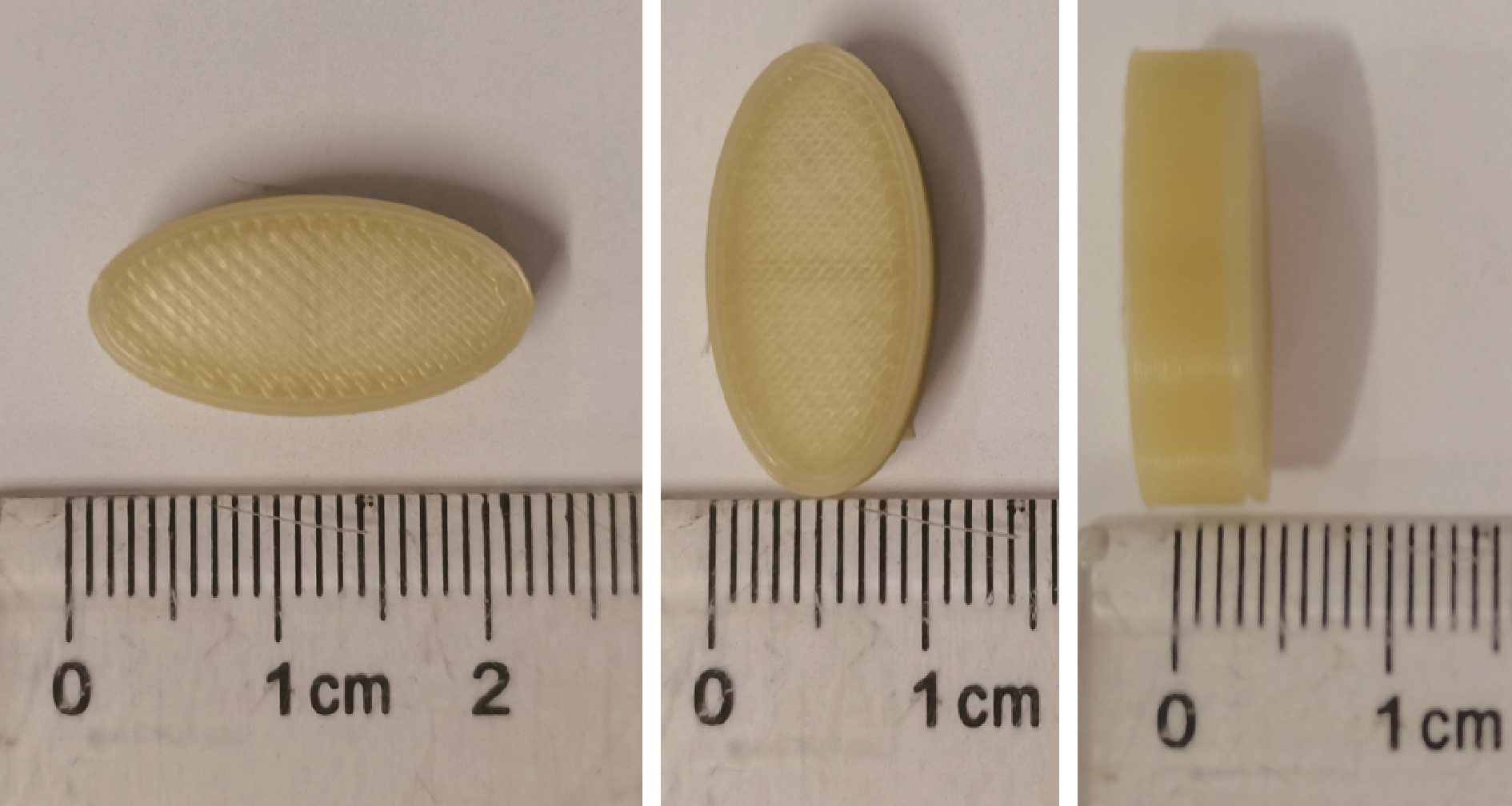
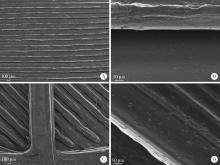
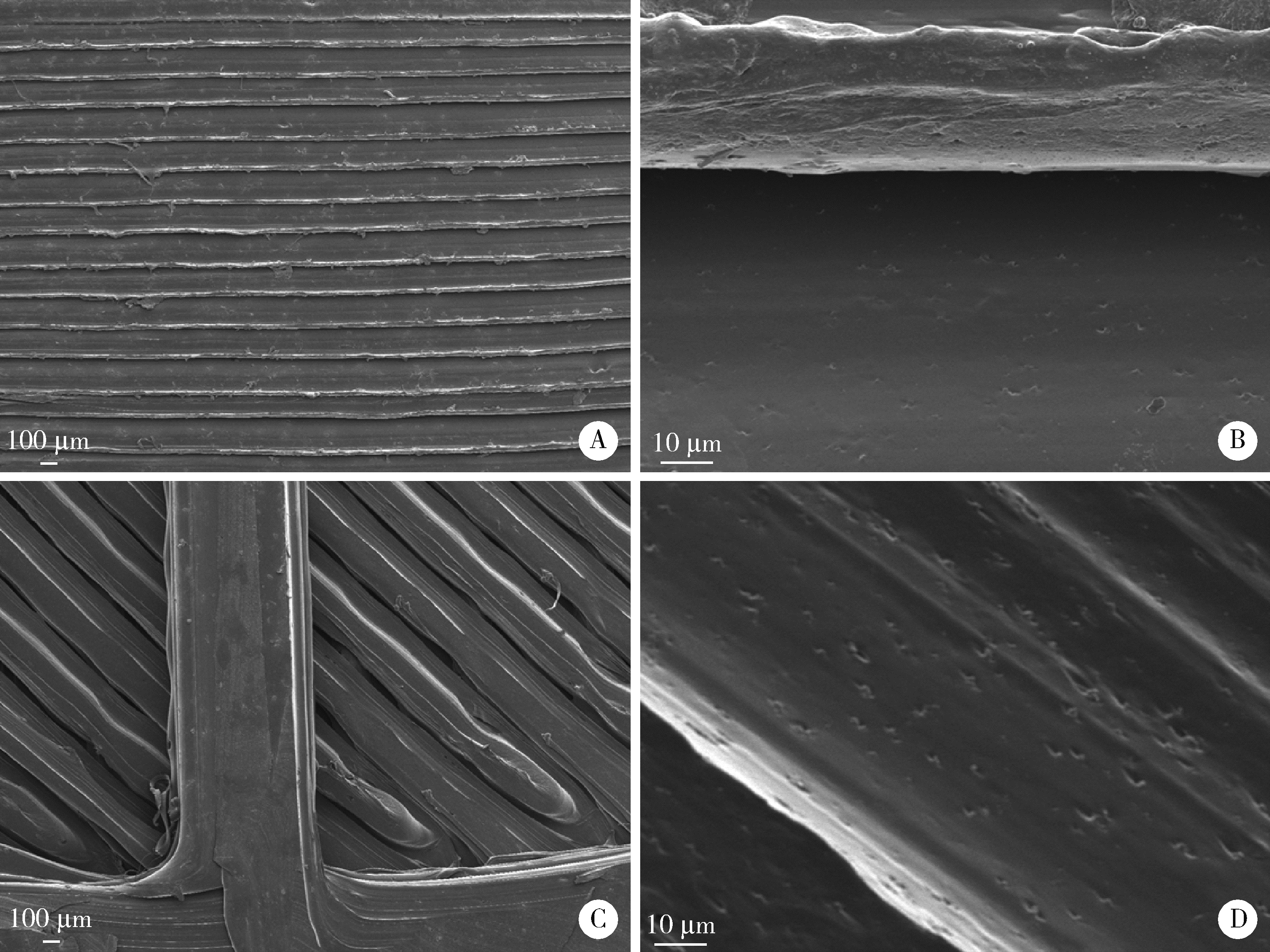
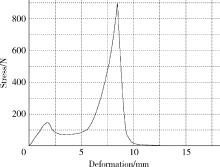
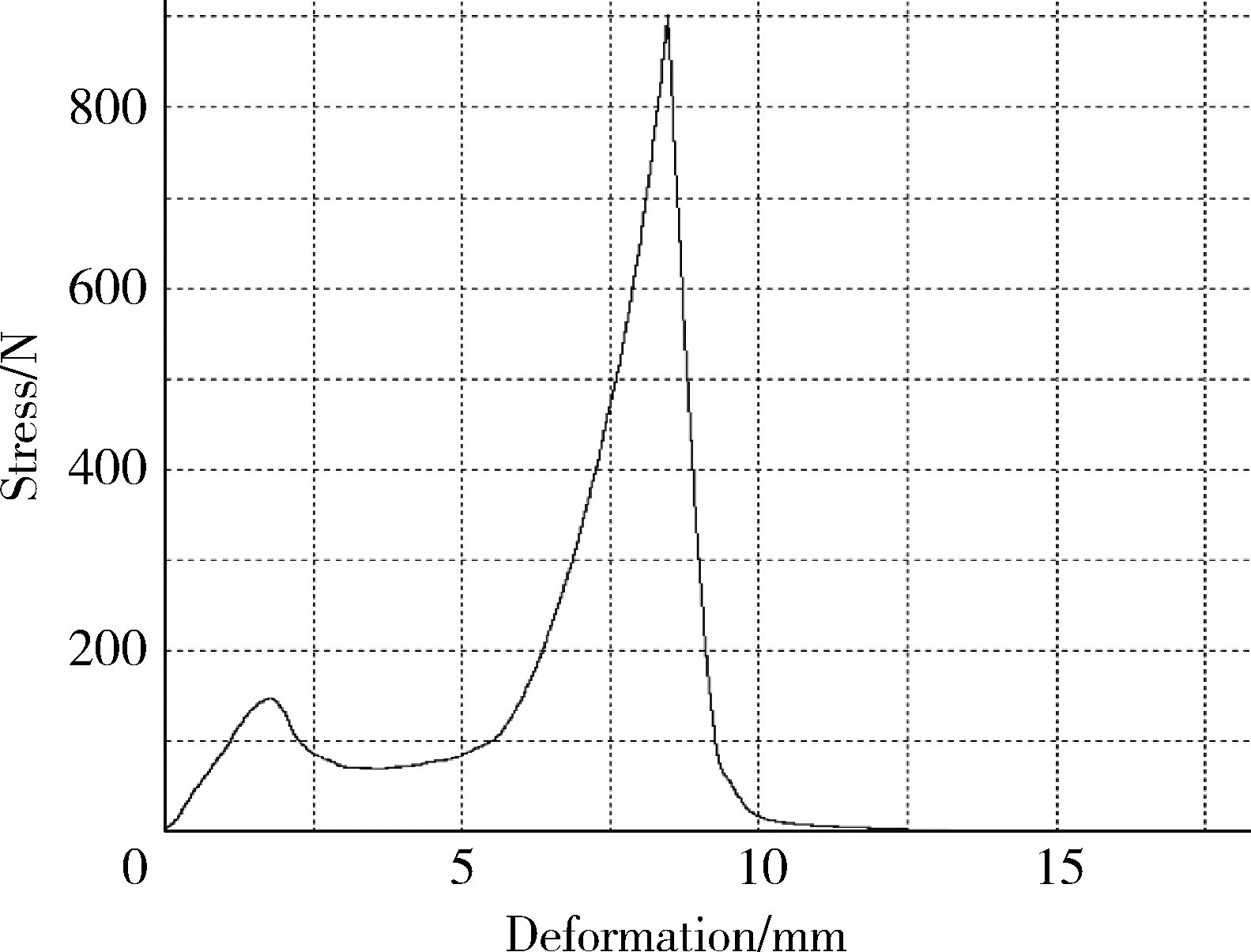
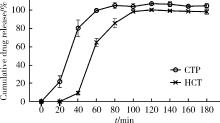
目的: 探究以熔融沉积成型(fused deposition modeling, FDM)3D打印技术制备治疗高血压复方片剂的可行性, 并对所制备的FDM 3D打印复方片剂进行相关的体外质量评价。方法: 以聚乙烯醇(polyvinyl alcohol, PVA)丝材作为辅料, 设计了具有两个独立隔室的椭圆形片剂(长轴20 mm, 短轴10 mm, 高5 mm), 其层高为0.2 mm, 外壳厚为1.2 mm, 顶和底厚均为0.6 mm, 两个隔室间的隔断厚为0.6 mm。使用FDM 3D打印机进行打印; 以卡托普利(captopril, CTP)和氢氯噻嗪(hydrochlorothiazide, HCT)为模型药物, 将其分别填充在片剂的两个隔室内。以扫描电镜观察制剂的外观形态, 考察制剂的质量差异和硬度, 以高效液相色谱法测定制剂中的药物含量, 并用溶出仪对制剂的体外释药行为进行表征。结果: 所制备的FDM 3D打印复方片剂均形态良好, 无打印缺陷; 平均质量为(644.3±6.55) mg, 其中CTP含量为(52.3±0.26) mg, HCT含量为(49.6±0.74) mg。观察到CTP和HCT在体外的延迟释放, 延迟释药时间分别为20 min和40 min, 释药70%的时间分别在30 min和60 min内。结论: 采用FDM 3D打印技术成功制备了CTP和HCT复方片剂, 并且所打印的复方片剂质量良好。
中图分类号:
- R944.4
| 1 |
Tan DK , Maniruzzaman M , Nokhodchi A . Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery[J]. Pharmaceutics, 2018, 10 (4): 203.
doi: 10.3390/pharmaceutics10040203 |
| 2 |
Okafor-Muo OL , Hassanin H , Kayyali R , et al. 3D printing of solid oral dosage forms: Numerous challenges with unique opportunities[J]. J Pharm Sci, 2020, 109 (12): 3535- 3550.
doi: 10.1016/j.xphs.2020.08.029 |
| 3 |
Brambilla CRM , Okafor-Muo OL , Hassanin H , et al. 3D printing of oral solid formulations: A systematic review[J]. Pharmaceutics, 2021, 13 (3): 358.
doi: 10.3390/pharmaceutics13030358 |
| 4 |
Melocchi A , Parietti F , Loreti G , et al. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs[J]. J Drug Deliv Sci Technol, 2015, 30 (Part B): 360- 367.
doi: 10.1016/j.jddst.2015.07.016 |
| 5 |
Lu J , Lu Y , Wang X , et al. Prevalence, awareness, treatment, and control of hypertension in China: Data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project)[J]. Lancet, 2017, 390 (10112): 2549- 2558.
doi: 10.1016/S0140-6736(17)32478-9 |
| 6 | 国家卫生计生委合理用药专家委员会, 中国医师协会高血压专业委员会. 高血压合理用药指南(第2版)[J]. 中国医学前沿杂志, 2017, 9 (7): 28- 126. |
| 7 |
An J , Derington CG , Luong T , et al. Fixed-dose combination medications for treating hypertension: A review of effectiveness, safety, and challenges[J]. Curr Hypertens Rep, 2020, 22 (11): 95.
doi: 10.1007/s11906-020-01109-2 |
| 8 | 国家药典委员会. 中华人民共和国药典(二部)[M]. 北京: 中国医药科技出版社, 2020: 963 |
| 9 | 国家药典委员会. 中华人民共和国药典(四部)[M]. 北京: 中国医药科技出版社, 2020: 112, 137, 478. |
| 10 |
Ghanizadeh Tabriz A , Nandi U , Hurt AP , et al. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment[J]. Int J Pharm, 2021, 593, 120147.
doi: 10.1016/j.ijpharm.2020.120147 |
| 11 | Charoenying T , Patrojanasophon P , Ngawhirunpat T , et al. Fabrication of floating capsule-in-3D-printed devices as gastro-retentive delivery systems of amoxicillin[J]. J Drug Deliv Sci Technol, 2020, 55, 100393. |
| 12 |
Wang Y , Sun L , Mei Z , et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma[J]. Materials & Design, 2020, 186, 108336.
doi: 10.1016/j.matdes.2019.108336 |
| 13 |
Kempin W , Domsta V , Grathoff G , et al. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug[J]. Pharm Res, 2018, 35 (6): 124.
doi: 10.1007/s11095-018-2405-6 |
| 14 |
Homaee Borujeni S , Mirdamadian SZ , Varshosaz J , et al. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile[J]. Cellulose, 2020, 27, 1573- 1589.
doi: 10.1007/s10570-019-02881-4 |
| 15 |
Fuenmayor E , Forde M , Healy AV , et al. Comparison of fused filament fabrication to direct compression and injection molding in the manufacture of oral tablets[J]. Int J Pharm, 2019, 558, 328- 340.
doi: 10.1016/j.ijpharm.2019.01.013 |
| 16 |
Yang Y , Wang H , Li H , et al. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release[J]. Eur J Pharm Sci, 2018, 115, 11- 18.
doi: 10.1016/j.ejps.2018.01.005 |
| 17 |
Mazzanti V , Malagutti L , Mollica F . FDM 3D printing of polymers containing natural fillers: A review of their mechanical properties[J]. Polymers (Basel), 2019, 11 (7): 1094.
doi: 10.3390/polym11071094 |
| 18 | Sadia M , Sosnicka A , Arafat B , et al. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets[J]. Int J Pharm, 2016, 513 (1/2): 659- 668. |
| 19 |
Melocchi A , Uboldi M , Briatico-Vangosa F , et al. The ChronotopicTM system for pulsatile and colonic delivery of active molecules in the era of precision medicine: Feasibility by 3D printing via fused deposition modeling (FDM)[J]. Pharmaceutics, 2021, 13 (5): 759.
doi: 10.3390/pharmaceutics13050759 |
| 20 | The U.S. Food and Drug Administration. FDA-approved drugs: Captopril (018343): 2. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020504s026lbl.pdf. |
| 21 | The U.S. Food and Drug Administration. FDA-approved drugs: Hydrochlorothiazide (084324): 2. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018343s087lbl.pdf. |
| [1] | 陈楚云,孙蓬飞,赵静,贾佳,范芳芳,王春燕,李建平,姜一梦,霍勇,张岩. 北京社区人群促红细胞生成素相关因素及其与10年心血管疾病风险的关系[J]. 北京大学学报(医学版), 2023, 55(6): 1068-1073. |
| [2] | 开地尔娅·阿不都热合曼,张荣赓,钱浩楠,邹振洋,丹尼娅·叶斯涛,范田园. 个性化剂量熔融沉积成型3D打印茶碱片剂的制备和体外评价[J]. 北京大学学报(医学版), 2022, 54(6): 1202-1207. |
| [3] | 梁喆,范芳芳,张岩,秦献辉,李建平,霍勇. 中国高血压人群中H型高血压的比率和特征及与美国人群的比较[J]. 北京大学学报(医学版), 2022, 54(5): 1028-1037. |
| [4] | 马麟,吴静依,李双成,李鹏飞,张路霞. 抗高血压药物对二氧化氮长期暴露与慢性肾脏病关联的修饰效应[J]. 北京大学学报(医学版), 2022, 54(5): 1047-1055. |
| [5] | 皇甫宇超,杜依青,于路平,徐涛. 原发性醛固酮增多症术后高血压未治愈的危险因素[J]. 北京大学学报(医学版), 2022, 54(4): 686-691. |
| [6] | 陈迪,徐翔宇,汪明睿,李芮,臧根奥,张悦,钱浩楠,闫光荣,范田园. 熔融沉积成型3D打印盐酸维拉帕米胃漂浮制剂的制备与体外评价[J]. 北京大学学报(医学版), 2021, 53(2): 348-354. |
| [7] | 杨航,杨林承,张瑞涛,凌云鹏,葛庆岗. 合并高血压、冠心病、糖尿病的新型冠状病毒肺炎患者发生病死的危险因素分析[J]. 北京大学学报(医学版), 2020, 52(3): 420-424. |
| [8] | 郑鸿尘,薛恩慈,王雪珩,陈曦,王斯悦,黄辉,江锦,叶莺,黄春兰,周筠,高文静,余灿清,吕筠,吴小玲,黄小明,曹卫华,严延生,吴涛,李立明. 基于大家系设计的静息心率与常见慢性病双表型遗传度估计[J]. 北京大学学报(医学版), 2020, 52(3): 432-437. |
| [9] | 孟文颖,黄琬桐,张杰,焦明远,金蕾,靳蕾. 孕早期血清维生素E水平与妊娠期高血压疾病发病风险的关系[J]. 北京大学学报(医学版), 2020, 52(3): 470-478. |
| [10] | 刘颖,曾祥柱,王筝,张函,王希林,袁慧书. 三维动脉自旋标记技术评价抑郁合并高血压患者脑血流灌注[J]. 北京大学学报(医学版), 2019, 51(2): 260-264. |
| [11] | 刘雪芹, 闫辉, 邱建星, 张春雨, 齐建光, 张欣, 肖慧捷, 杨艳玲, 陈永红, 杜军保. 甲基丙二酸尿症相关肺高血压临床特点与基因突变[J]. 北京大学学报(医学版), 2017, 49(5): 768-777. |
| [12] | 单娇,李宏宇,刘国峰,杨玄,董伟,简伟研,邓芙蓉,郭新彪. 大气污染对中老年高血压和心脑血管疾病患者卫生服务需求的影响:基于 CHARLS数据的分析[J]. 北京大学学报(医学版), 2016, 48(3): 460-464. |
| [13] | 章湖洋,简伟研,方海. 新型农村合作医疗的高血压患者门诊费用对住院费用的替代效应[J]. 北京大学学报(医学版), 2016, 48(3): 472-477. |
| [14] | 杨莹超,刘国莉,周敬伟,胡浩,沈丹华. 妊娠合并嗜铬细胞瘤1例[J]. 北京大学学报(医学版), 2016, 48(2): 370-372. |
| [15] | 陈红涛, 王文英, 王津, 梁亚平, 王小婷, 侯光敏, 姬爱平. 不同高血压分级患者急性牙髓炎开髓治疗的风险评估[J]. 北京大学学报(医学版), 2016, 48(1): 89-93. |
|
||