北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (3): 451-458. doi: 10.19723/j.issn.1671-167X.2019.03.012
单中心大样本Epstein-Barr病毒相关性胃癌亚型的临床病理及分子特征分析
杨阳1,刘毅强2,王晓红3,季科1,李忠武2,白健4,杨爱蓉4,胡颖3,韩海勃3,李子禹1,步召德1,吴晓江1,张连海1△( ),季加孚1△(
),季加孚1△( )
)
- 北京大学肿瘤医院暨北京市肿瘤防治研究所,恶性肿瘤发病机制及转化研究教育部重点实验室, 1. 胃肠肿瘤中心
2. 病理科
3. 生物样本库, 北京 100142
4. 和瑞基因科技有限公司,北京 102206
Clinicopathological and molecular characteristics of Epstein-Barr virus associated gastric cancer: a single center large sample case investigation
Yang YANG1,Yi-qiang LIU2,Xiao-hong WANG3,Ke JI1,Zhong-wu LI2,Jian BAI4,Ai-rong YANG4,Ying HU3,Hai-bo HAN3,Zi-yu LI1,Zhao-de BU1,Xiao-jiang WU1,Lian-hai ZHANG1△( ),Jia-fu JI1△(
),Jia-fu JI1△( )
)
- 1. Department of Gastrointestinal Cancer Center
2. Department of Pathology
3. Department of Biobank, Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Laboratory of Genetics, Peking University Cancer Hospital & Institute, Beijing 100142, China
4. Berry Oncology Corporation, Beijing 102206, China
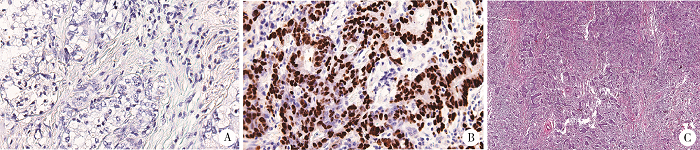
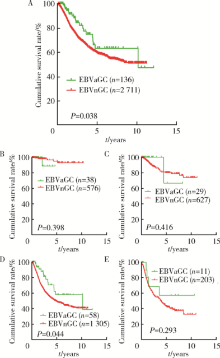
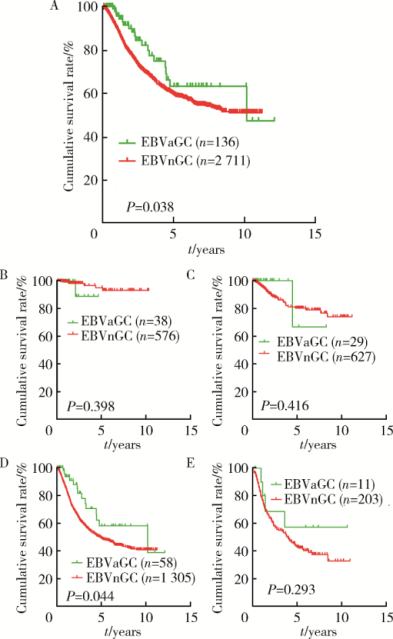
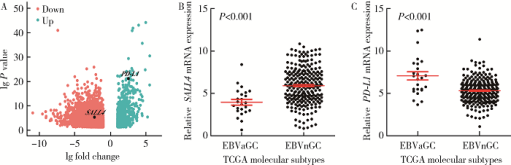
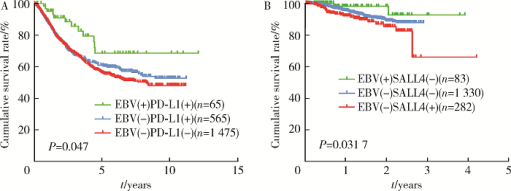
摘要: 目的 Epstein-Barr病毒相关性胃癌(Epstein-Barr virus associated gastric cancer, EBVaGC)与常见胃癌(非Epstein-Barr病毒相关性胃癌,Epstein-Barr virus non-associated gastric cancer, EBVnGC)不同,具有独特的临床病理特征,本研究采用单中心大样本探讨中国EBVaGC的临床病理及分子特征。方法 回顾分析2003—2018年北京大学肿瘤医院EBVaGC与EBVnGC两组患者的临床病理特征和预后。分析公共数据库胃癌数据集,筛选显著差异表达基因,并在本组病例中验证重要基因的表达及其与预后的相关性。结果 3 241例胃癌患者纳入研究,EBVaGC为163例,占总数的5.0%。与EBVnGC相比,EBVaGC男性常见,平均年龄低,多见于残胃癌,常为低分化腺癌、Lauren混合型,较少出现淋巴结转移,EBVaGC患者的5年生存率为63.2%,优于EBVnGC的59.6%(P<0.05)。为挖掘EBVaGC的分子特征,对癌症基因组图谱(The Cancer Genome Atlas, TCGA)胃癌数据集(n=240)进行分析,筛选到7 404个显著差异表达基因,涉及细胞增殖、凋亡、侵袭转移等功能,其中侵袭转移相关基因SALL4下调、免疫检查点相关基因PD-L1上调是EBVaGC重要的分子特征。大样本验证显示,SALL4在EBVaGC中多为阴性(1/92,1.1%,低于EBVnGC的303/1 727,17.5%),PD-L1在EBVaGC中多为阳性(81/110,73.6%,高于EBVnGC的649/2 350,27.6%),SALL4阴性和PD-L1阳性患者的预后较好。结论 EBVaGC作为独特的胃癌亚型,较少出现转移且预后良好,该亚型具有特征性分子背景,其中侵袭转移相关基因SALL4的下调以及免疫检查点相关基因PD-L1的上调是重要的分子特征。
中图分类号:
- R735.2
| [1] |
Horiuchi K, Mishima K, Ohsawa M , et al. Carcinoma of stomach and breast with lymphoid stroma: localisation of Epstein-Barr virus[J]. J Clin Pathol, 1994,47(6):538-540.
doi: 10.1136/jcp.47.6.538 |
| [2] |
Network CGAR . Comprehensive molecular characterization of gastric adenocarcinoma[J]. Nature, 2014,513(7517):202.
doi: 10.1038/nature13480 |
| [3] |
Baek DW, Kang BW, Hwang S , et al. Clinical significance of p53 protein expression, beta-catenin expression and HER2 expression for Epstein-Barr virus-associated gastric cancer[J]. Chonnam Med J, 2017,53(2):140-146.
doi: 10.4068/cmj.2017.53.2.140 |
| [4] |
Camargo MC, Kim WH, Chiaravalli AM , et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis[J]. Gut, 2014,63(2):236-243.
doi: 10.1136/gutjnl-2013-304531 |
| [5] |
Camargo M, Murphy G, Koriyama C , et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis[J]. Brit J Cancer, 2011,105(1):38.
doi: 10.1038/bjc.2011.215 |
| [6] |
Murphy G, Pfeiffer R, Camargo MC , et al. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location[J]. Gastroenterology, 2009,137(3):824-833.
doi: 10.1053/j.gastro.2009.05.001 |
| [7] |
Dong M, Wang HY, Zhao XX , et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma[J]. Hum Pathol, 2016,53:25-34.
doi: 10.1016/j.humpath.2016.02.007 |
| [8] | Myers RB, Kudlow JE, Grizzle WE . Expression of transforming growth factor-alpha, epidermal growth factor and the epidermal growth factor receptor in adenocarcinoma of the prostate and benign prostatic hyperplasia[J]. Mod Pathol, 1993,6(6):733-737. |
| [9] |
Yong KJ, Gao C, Lim JS , et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma[J]. N Engl J Med, 2013,368(24):2266-2276.
doi: 10.1056/NEJMoa1300297 |
| [10] |
Harn HJ, Chang JY, Wang MW , et al. Epstein-Barr virus-asso-ciated gastric adenocarcinoma in Taiwan[J]. Hum Pathol, 1995,26(3):267-271.
doi: 10.1016/0046-8177(95)90056-X |
| [11] |
Qiu K, Tomita Y, Hashimoto M , et al. Epstein-Barr virus in gastric carcinoma in Suzhou, China and Osaka, Japan: Association with clinico-pathologic factors and HLA-subtype[J]. Int J Can-cer, 1997,71(2):155-158.
doi: 10.1002/(ISSN)1097-0215 |
| [12] |
Liu S, Zhao Z, Han L , et al. Epstein-Barr virus infection in gastric remnant carcinoma and recurrent gastric carcinoma in Qingdao of Northern China[J]. PLoS One, 2016,11(2):e0148342.
doi: 10.1371/journal.pone.0148342 |
| [13] |
Sukawa Y, Yamamoto H, Nosho K , et al. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer[J]. World J Gastroenterol, 2012,18(45):6577-6586.
doi: 10.3748/wjg.v18.i45.6577 |
| [14] |
Kaizaki Y, Hosokawa O, Sakurai S , et al. Epstein-Barr virus-associated gastric carcinoma in the remnant stomach: de novo and metachronous gastric remnant carcinoma[J]. J Gastroenterol, 2005,40(6):570-577.
doi: 10.1007/s00535-005-1590-3 |
| [15] |
Zhang L, Xu Z, Xu X , et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis[J]. Oncogene, 2014,33(48):5491-5500.
doi: 10.1038/onc.2013.495 |
| [16] |
Kim ST, Cristescu R, Bass AJ , et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metasta-tic gastric cancer[J]. Nat Med, 2018,24(9):1449-1458.
doi: 10.1038/s41591-018-0101-z |
| [1] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [2] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [3] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [4] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [5] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [6] | 曾媛媛,谢云,陈道南,王瑞兰. 脓毒症患者发生正常甲状腺性病态综合征的相关因素[J]. 北京大学学报(医学版), 2024, 56(3): 526-532. |
| [7] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [8] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [9] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [10] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [11] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [12] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [13] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [14] | 赖玉梅,李忠武,李欢,吴艳,时云飞,周立新,楼雨彤,崔传亮. 68例肛管直肠黏膜黑色素瘤临床病理特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 262-269. |
| [15] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
|
||