北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (2): 353-361. doi: 10.19723/j.issn.1671-167X.2020.02.025
结直肠间质瘤临床病理特征及预后分析
王文鹏1,王捷夫1,胡均1,王俊锋1,刘嘉1,孔大陆1,△( ),李健2
),李健2
- 1. 大连医科大学附属第一医院放射科, 辽宁大连 116011
2. 北京大学医学部医学技术研究院, 北京 100191
Clinicopathological features and prognosis of colorectal stromal tumor
Wen-peng WANG1,Jie-fu WANG1,Jun HU1,Jun-feng WANG1,Jia LIU1,Da-lu KONG1,△( ),Jian LI2
),Jian LI2
- 1. Department of Radiology, the First Affiliated Hospital of Dalian Medical University, Dalian 116011, Liaoning, China
2. Institute of Medical Technology, Peking University Health Science Center, Beijing 100191, China
摘要:
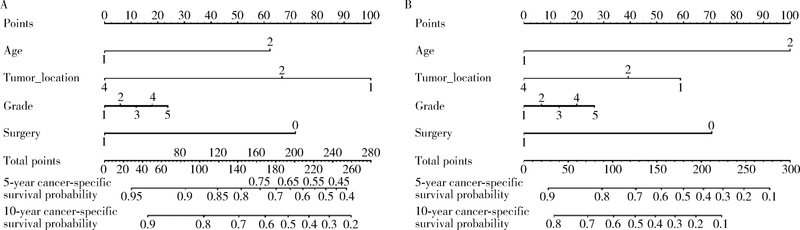
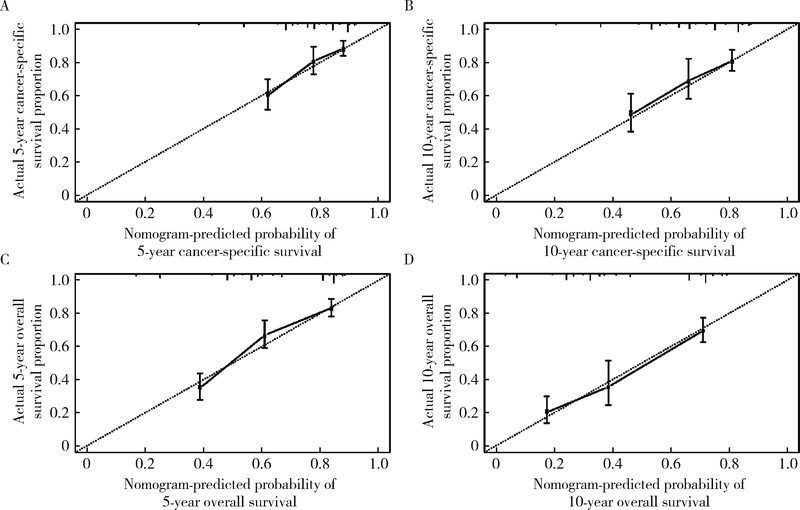
目的 探究结直肠间质瘤预后相关因素,并通过列线图预测该肿瘤生存概率,为指导临床评估预后提供依据.方法: 通过监测流行病学和最终结果(surveillance, epidemiology, and end results, SEER)数据库获取1992年1月至2015年12月结直肠间质瘤临床病理及预后相关资料,对入组患者进行生存分析,将分析得到的独立预后因素绘制成列线图,之后采用校准曲线评估列线图预测生存准确性.结果: 546例结直肠间质瘤患者被纳入研究.中位发病年龄64岁,区域淋巴结转移率9.4%.546例患者多因素生存分析显示发病年龄 > 64岁,未婚/离婚,结肠间质瘤(与直肠间质瘤相比),非手术治疗,组织分化级别高,区域淋巴结转移及远处转移具有更差的肿瘤特异性生存和总生存(P均<0.05), 美国东部地区诊治患者比西部地区患者具有更长的总生存时间(P = 0.027),以上独立预后因素预测肿瘤特异性生存率和总生存率的C指数分别为0.76(95%CI: 0.72-0.80)和0.75(95%CI: 0.72-0.78).在174例组织分化级别和肿瘤部位明确的患者中,影响肿瘤特异性生存和总生存的独立预后因素为年龄,组织分化级别和是否行手术治疗(P均<0.05),而肿瘤部位仅与肿瘤特异性生存显著相关(P = 0.041),未证实与总生存显著相关(P = 0.057),采用这4个预后影响因素预测546例患者肿瘤特异性生存率和总生存率的C指数分别是0.71(95%CI: 0.66-0.75)和0.73(95%CI: 0.70-0.77), 能较准确预测结直肠间质瘤患者总生存率.结论: 结直肠间质瘤预后受多个临床病理因素影响,列线图能为预测结直肠间质瘤患者生存率提供依据.
中图分类号:
- R735.3
| [1] | Nilsson B, Bumming P, Meis-Kindblom JM , et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden[J]. Cancer, 2005,103(4):821-829. |
| [2] | Parab TM , DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review[J]. J Gastrointest Oncol, 2019,10(1):144-154. |
| [3] | Kindblom LG, Remotti HE, Aldenborg F , et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal[J]. Am J Pathol, 1998,152(5):1259-1269. |
| [4] | Soreide K, Sandvik OM, Soreide JA , et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies[J]. Cancer Epidemiol, 2016,40:39-46. |
| [5] | Corbin KS, Kindler HL, Liauw SL . Considering the role of radiation therapy for gastrointestinal stromal tumor[J]. Onco Targets Ther, 2014,7:713-718. |
| [6] | Wu PC, Langerman A, Ryan CW , et al. Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era[J]. Surgery, 2003,134(4):656-665. |
| [7] | 杨朝纲, 熊斌 . 结直肠间质瘤诊治进展[J]. 实用癌症杂志, 2015,30(10):1575-1577. |
| [8] | Miettinen M, Lasota J, Sobin LH . Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1 765 cases with longterm follow-up[J]. Am J Surg Pathol, 2005,29(10):1373-1381. |
| [9] | Al-Thani H, El-Menyar A, Rasul KI , et al. Clinical presentation, management and outcomes of gastrointestinal stromal tumors[J]. Int J Surg, 2014,12(10):1127-1133. |
| [10] | Cao H, Zhang Y, Wang M , et al. Prognostic analysis of patients with gastrointestinal stromal tumors: a single unit experience with surgical treatment of primary disease[J]. Chin Med J (Engl), 2010,123(2):131-136. |
| [11] | Varshney VK, Gupta RK, Saluja SS , et al. Analysis of clinicopathological and immunohistochemical parameters and correlation of outcomes in gastrointestinal stromal tumors[J]. Indian J Can-cer, 2019,56(2):135-143. |
| [12] | Ghanem N, Altehoefer C, Furtwangler A , et al. Computed tomography in gastrointestinal stromal tumors[J]. Eur Radiol, 2003,13(7):1669-1678. |
| [13] | Tateishi U, Hasegawa T, Satake M , et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality[J]. J Comput Assist Tomogr, 2003,27(5):792-798. |
| [14] | Gaitanidis A, El Lakis M, Alevizakos M , et al. Predictors of lymph node metastasis in patients with gastrointestinal stromal tumors (GISTs)[J]. Langenbecks Arch Surg, 2018,403(5):599-606. |
| [15] | Tokunaga M, Ohyama S, Hiki N , et al. Incidence and prognostic value of lymph node metastasis on c-Kit-positive gastrointestinal stromal tumors of the stomach[J]. Hepatogastroenterology, 2011,58(109):1224-1228. |
| [16] | Zhu R, Liu F, Grisotti G , et al. Distinctive features of gastrointestinal stromal tumors arising from the colon and rectum[J]. J Gastrointest Oncol, 2018,9(2):231-240. |
| [17] | 黄湘辉, 裘科跃, 袁旦平 , 等. 102例胃肠间质瘤外科手术疗效及预后影响因素分析[J]. 解放军医学院学报, 2019,40(7):655-659. |
| [18] | Ge XY, Lei LW, Ge F , et al. Analysis of risk factors of gastrointestinal stromal tumors in different age groups based on SEER database[J]. Scand J Gastroenterol, 2019,54(4):480-484. |
| [19] | Buja A, Lago L, Lago S , et al. Marital status and stage of cancer at diagnosis: A systematic review[J]. Eur J Cancer Care (Engl), 2018,27 (2017-08-29)[2019-10-30]. |
| [20] | Li J, Ye Y, Wang J , et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor[J]. Chinese Journal of Cancer Research, 2017,29(4):281-293. |
| [21] | Wada R, Arai H, Kure S , et al. "Wild type" GIST: Clinicopathological features and clinical practice[J]. Pathol Int, 2016,66(8):431-437. |
| [22] | 艾力·赛丁, 艾克拜尔·艾力, 张成 , 等. DOG1在胃肠道间质瘤中的表达及其临床意义[J]. 现代肿瘤医学, 2016,24(21):3418-3421. |
| [23] | 李超亿, 梁小波, 马俊杰 , 等. C-kit与血小板源性生长因子受体基因突变特征与胃肠间质瘤患者预后的关系[J]. 中华胃肠外科杂志, 2012,15(3):271-275. |
| [1] | 刘园梅, 傅义程, 郝靖欣, 张福春, 刘慧琳. 老年髋部骨折患者住院期间发生术后心力衰竭的列线图预测模型的构建及验证[J]. 北京大学学报(医学版), 2024, 56(5): 874-883. |
| [2] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [3] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [4] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [5] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [6] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [7] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [8] | 曾媛媛,谢云,陈道南,王瑞兰. 脓毒症患者发生正常甲状腺性病态综合征的相关因素[J]. 北京大学学报(医学版), 2024, 56(3): 526-532. |
| [9] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [10] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [11] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [12] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [13] | 毛海,张帆,张展奕,颜野,郝一昌,黄毅,马潞林,褚红玲,张树栋. 基于MRI前列腺腺体相关参数构建腹腔镜前列腺癌术后尿失禁的预测模型[J]. 北京大学学报(医学版), 2023, 55(5): 818-824. |
| [14] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [15] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
|
||