北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (5): 821-827. doi: 10.19723/j.issn.1671-167X.2020.05.005
纳米二氧化钛亚急性经口暴露对大鼠氧化/抗氧化生物标志和炎性因子的影响
- 北京大学公共卫生学院劳动卫生与环境卫生学系, 北京 100191
Influence of oxidative/antioxidative biomarkers and inflammatory cytokines on rats after sub-acute orally administration of titanium dioxide nanoparticles
Di ZHOU,Zhang-jian CHEN,Gui-ping HU,Teng-long YAN,Chang-mao LONG,Hui-min FENG,Guang JIA( )
)
- Department of Occupational and Environmental Health Sciences, Peking University School of Public Health, Beijing 100191, China
摘要:
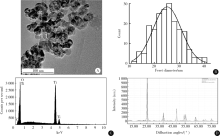
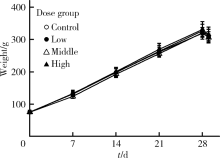
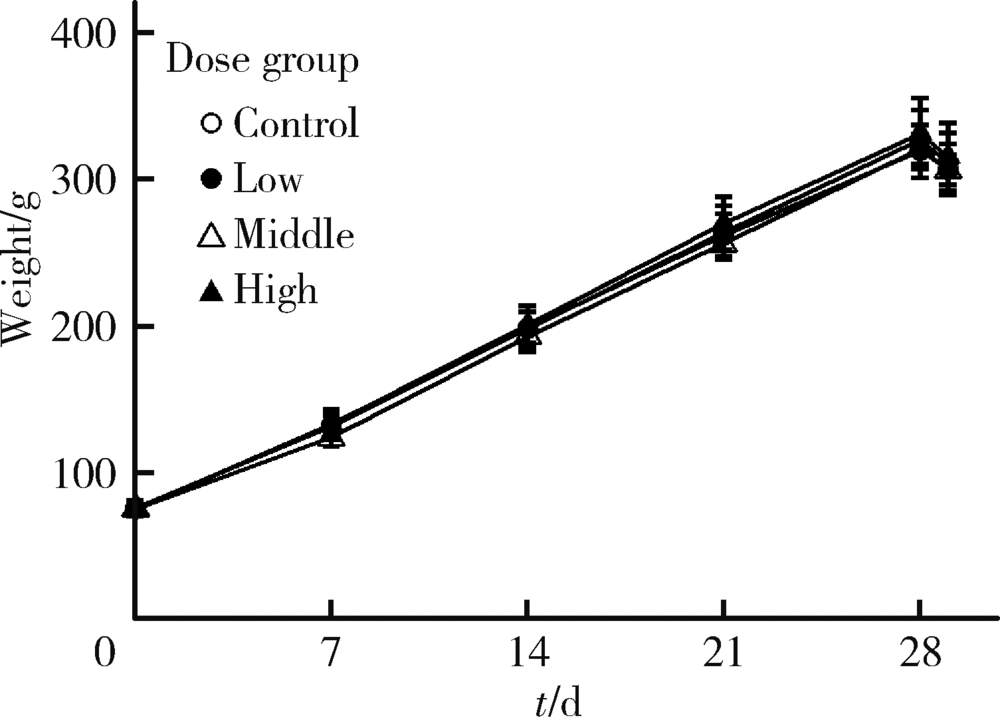
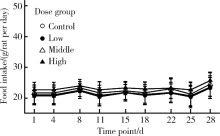
目的:评估纳米二氧化钛(titanium dioxide, TiO2)亚急性经口暴露对大鼠肝、小肠、大肠及血液等组织、脏器氧化/抗氧化生物标志和炎性因子的影响。方法:将24只4周龄清洁级Sprague Dawley(SD)雄性大鼠按体质量随机分为对照、低剂量、中剂量和高剂量4组,每组6只,以纳米TiO2每日灌胃,染毒剂量分别为0、2、10和50 mg/kg体质量,持续28 d。记录大鼠进食量、体质量和异常表现等一般情况,末次染毒后禁食12 h,取大鼠腹主动脉血,离心收集血清,取肝、小肠、大肠组织制备匀浆。采用酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)及微板比色法测定氧化/抗氧化生物标志:总超氧化物歧化酶(superoxide dismutase, SOD)、还原型谷胱甘肽(glutathione, GSH)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)、总巯基(total mercapto, T-SH)、氧化型谷胱甘肽(glutathione disulfide, GSSG)和丙二醛(malomdialdehvde, MDA);测定炎性因子:白细胞介素-6(interleukin 6, IL-6)和肿瘤坏死因子-α(tumor necrosis factor alpha, TNF-α)。结果:与对照组相比,各组大鼠体质量、进食量、脏器系数均未见明显差异,血清、肝、小肠、大肠中GSH、GSH-Px、T-SH和IL-6无显著改变。与各自对照组相比,高剂量组大鼠血清中SOD活性显著上升,中、高剂量组小肠中GSSG浓度显著升高,低、高剂量组肝MDA浓度显著升高,中剂量组和高剂量组肝TNF-α浓度显著升高。结论:纳米TiO2亚急性经口暴露可造成大鼠血液组织抗氧化酶活性增加,肝和小肠氧化产物增加,肝脏炎性因子水平增高,肝对纳米TiO2经口暴露毒性最为敏感,随后依次为小肠和血液组织,大肠最不敏感。
中图分类号:
- R155.3
| [1] |
Warheit DB, Donner EM. Risk assessment strategies for nanoscale and fine-sized titanium dioxide particles:Recognizing hazard and exposure issues[J]. Food Chem Toxicol, 2015,85:138-147.
doi: 10.1016/j.fct.2015.07.001 pmid: 26362081 |
| [2] |
Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products[J]. Environ Sci Technol, 2012,46(4):2242-2250.
doi: 10.1021/es204168d pmid: 22260395 |
| [3] |
Yang Y, Doudrick K, Bi X, et al. Characterization of food-grade titanium dioxide: the presence of nanosized particles[J]. Environ Sci Technol, 2014,48(11):6391-6400.
doi: 10.1021/es500436x pmid: 24754874 |
| [4] |
Robichaud CO, Uyar AE, Darby MR, et al. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment[J]. Environ Sci Technol, 2009,43(12):4227-4233.
doi: 10.1021/es8032549 pmid: 19603627 |
| [5] |
Rompelberg C, Heringa MB, van Donkersgoed GC, et al. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population[J]. Nanotoxicology, 2016,10(10):1404-1414.
doi: 10.1080/17435390.2016.1222457 pmid: 27619007 |
| [6] |
Scherbart AM, Langer J, Bushmelev A, et al. Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms[J]. Part Fibre Toxicol, 2011,8(31):1-19.
doi: 10.1186/1743-8977-8-1 |
| [7] |
Katsumiti A, Berhanu D, Howard KT, et al. Cytotoxicity of TiO2 nanoparticles to mussel hemocytes and gill cells in vitro: influence of synjournal method, crystalline structure, size and additive[J]. Nanotoxicology, 2015,9(5):543-553.
doi: 10.3109/17435390.2014.952362 pmid: 25188678 |
| [8] |
Takaki K, Higuchi Y, Hashii M, et al. Induction of apoptosis associated with chromosomal DNA fragmentation and caspase-3 activation in leukemia L1210 cells by TiO2 nanoparticles[J]. J Biosci Bioeng, 2014,117(1):129-133.
doi: 10.1016/j.jbiosc.2013.06.003 |
| [9] |
Zhang Z, Liang ZC, Zhang JH, et al. Nano-sized TiO2 (nTiO2) induces metabolic perturbations in Physarum polycephalum macroplasmodium to counter oxidative stress under dark conditions[J]. Ecotoxicol Environ Saf, 2018,154:108-117.
doi: 10.1016/j.ecoenv.2018.02.012 pmid: 29454986 |
| [10] |
Ammendolia MG, Iosi F, Maranghi F, et al. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells[J]. Food Chem Toxicol, 2017,102:63-75.
doi: 10.1016/j.fct.2017.01.031 pmid: 28159593 |
| [11] |
Bachler G, von Goetz N, Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles[J]. Nanotoxicology, 2015,9(3):373-380.
doi: 10.3109/17435390.2014.940404 pmid: 25058655 |
| [12] | Huybrechts I, Sioena I, Boon PE, et al. Long-term dietary exposure to different food colours in young children living in different European countries[J]. EFSA Support Publ, 2010,7(5):53E. |
| [13] |
Cui YL, Gong XL, Duan YM, et al. Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles[J]. J Hazard Mater, 2010,183(1-3):874-880.
doi: 10.1016/j.jhazmat.2010.07.109 pmid: 20724067 |
| [14] |
He J, Wang T, Wang P, et al. A novel mechanism underlying the susceptibility of neuronal cells to nitric oxide: the occurrence and regulation of protein S-nitrosylation is the checkpoint[J]. J Neurochem, 2007,102(6):1863-1874.
doi: 10.1111/j.1471-4159.2007.04651.x pmid: 17767703 |
| [15] |
Lugrin J, Rosenblatt VN, Parapanov R, et al. The role of oxidative stress during inflammatory processes[J]. Biol Chem, 2014,395(2):203-230.
doi: 10.1515/hsz-2013-0241 pmid: 24127541 |
| [16] |
Lei XG, Zhu JH, Cheng WH, et al. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications[J]. Physiol Rev, 2016,96(1):307-364.
doi: 10.1152/physrev.00010.2014 pmid: 26681794 |
| [17] |
Huang XZ, Lan YW, Liu ZK, et al. Salinity mediates the toxic effect of nano-TiO2 on the juvenile olive flounder Paralichthys olivaceus [J]. Sci Total Environ, 2018, 640-641:726-735.
doi: 10.1016/j.scitotenv.2018.05.350 pmid: 29879661 |
| [18] |
Sentellas S, Morales IO, Zanuy M, et al. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress[J]. Toxicol Vitr, 2014,28(5):1006-1015.
doi: 10.1016/j.tiv.2014.04.017 |
| [19] |
Hu HL, Guo Q, Wang CL, et al. Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice[J]. J Appl Toxicol, 2015,35(10):1122-1132.
doi: 10.1002/jat.3150 pmid: 25826740 |
| [20] |
Shukla RK, Kumar A, Vallabani NVS, et al. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice[J]. Nanomedicine, 2014,9(9):1423-1434.
doi: 10.2217/NNM.13.100 |
| [21] |
Gornati R, Longo A, Rossi F, et al. Effects of titanium dioxide nanoparticle exposure in Mytilus galloprovincialis gills and digestive gland[J]. Nanotoxicology, 2016,10(6):807-817.
doi: 10.3109/17435390.2015.1132348 pmid: 26846715 |
| [22] |
Chen ZJ, Wang Y, Zhuo L, et al. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration[J]. Toxicol Lett, 2015,239(2):123-130.
doi: 10.1016/j.toxlet.2015.09.013 pmid: 26387441 |
| [23] |
Sycheva LP, Zhurkov VS, Iurchenko VV, et al. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo[J]. Mutat Res, 2011,726(1):8-14.
doi: 10.1016/j.mrgentox.2011.07.010 pmid: 21871579 |
| [1] | 张展奕,张帆,颜野,曹财广,李长剑,邓绍晖,孙悦皓,黄天亮,管允鹤,李楠,陆敏,胡振华,张树栋. 近红外荧光靶向探针用于前列腺神经血管束术中成像[J]. 北京大学学报(医学版), 2023, 55(5): 843-850. |
| [2] | 史佳琪,马莺,张奕,陈章健,贾光. 纳米二氧化钛颗粒对人肝癌细胞HepG2中circRNA表达谱的影响[J]. 北京大学学报(医学版), 2023, 55(3): 392-399. |
| [3] | 孟令玮,李雪,高胜寒,李悦,曹瑞涛,张毅,潘韶霞. 三种方法建立大鼠种植体周炎模型的比较[J]. 北京大学学报(医学版), 2023, 55(1): 22-29. |
| [4] | 张家赫,史佳琪,陈章健,贾光. 基于人消化道微生态体外模拟系统观察纳米二氧化钛对肠道菌群的影响[J]. 北京大学学报(医学版), 2022, 54(3): 468-476. |
| [5] | 何伟,杨思雯,陈娟,朱晓俊,陈志忠,马文军. 275 nm和310 nm紫外线对去卵巢骨质疏松大鼠骨代谢的影响[J]. 北京大学学报(医学版), 2022, 54(2): 236-243. |
| [6] | 陈章健,韩硕,郑湃,贾光. 锐钛矿型纳米二氧化钛经口暴露90天对Sprague-Dawley大鼠血常规指标的影响[J]. 北京大学学报(医学版), 2021, 53(6): 1205-1208. |
| [7] | 王贵红,左婷,李然,左正才. 瑞巴派特在大鼠痛风性关节炎急性发作中的作用[J]. 北京大学学报(医学版), 2021, 53(4): 716-720. |
| [8] | 尹雪倩, 张晓玄, 文婧, 刘思奇, 刘欣然, 周若宇, 王军波. 荞麦、燕麦、豌豆复配对糖尿病大鼠血糖的影响[J]. 北京大学学报(医学版), 2021, 53(3): 447-452. |
| [9] | 白枫,何倚帆,牛亚楠,杨若娟,曹静. 超细颗粒物对大鼠离体灌注心脏功能的影响[J]. 北京大学学报(医学版), 2021, 53(2): 240-245. |
| [10] | 陈章健,韩硕,郑湃,周淑佩,贾光. 纳米二氧化钛与葡萄糖亚慢性联合经口暴露对幼年大鼠血清叶酸和维生素B12水平的影响[J]. 北京大学学报(医学版), 2020, 52(3): 451-456. |
| [11] | 韩硕,陈章健,周迪,郑湃,张家赫,贾光. 纳米二氧化钛经口暴露90天对大鼠粪便代谢组的影响[J]. 北京大学学报(医学版), 2020, 52(3): 457-463. |
| [12] | 白珊珊,莫思怡,徐啸翔,刘云,谢秋菲,曹烨. 大鼠咬合干扰致口颌面痛敏的自我赏罚实验行为学特点[J]. 北京大学学报(医学版), 2020, 52(1): 51-57. |
| [13] | 何姣,袁戈恒,张俊清,郭晓蕙. 早期糖尿病周围神经病变大鼠模型的建立[J]. 北京大学学报(医学版), 2019, 51(6): 1150-1154. |
| [14] | 王伟,侯进,黄文强. 运动导致兴奋脑区组织液流动一过性加速[J]. 北京大学学报(医学版), 2019, 51(2): 206-209. |
| [15] | 段淑敏,张永亮,王云. 纳米二氧化钛与脂多糖对小鼠肝脏抗氧化性能的影响[J]. 北京大学学报(医学版), 2018, 50(3): 395-400. |
|
||