北京大学学报(医学版) ›› 2022, Vol. 54 ›› Issue (4): 644-651. doi: 10.19723/j.issn.1671-167X.2022.04.010
泌尿肿瘤免疫检查点抑制剂相关性肌炎的临床特征
应沂岑,唐琦*( ),杨恺惟,米悦,范宇,虞巍,宋毅,何志嵩,周利群,李学松*(
),杨恺惟,米悦,范宇,虞巍,宋毅,何志嵩,周利群,李学松*( )
)
- 北京大学第一医院泌尿外科,北京大学泌尿外科研究所,国家泌尿、男性生殖系肿瘤研究中心,北京 100034
Clinical features of immune checkpoint inhibitor-related myositis in patients with urological cancer
Yi-cen YING,Qi TANG*( ),Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI*(
),Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI*( )
)
- Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Cancer Center, Beijing 100034, China
摘要:
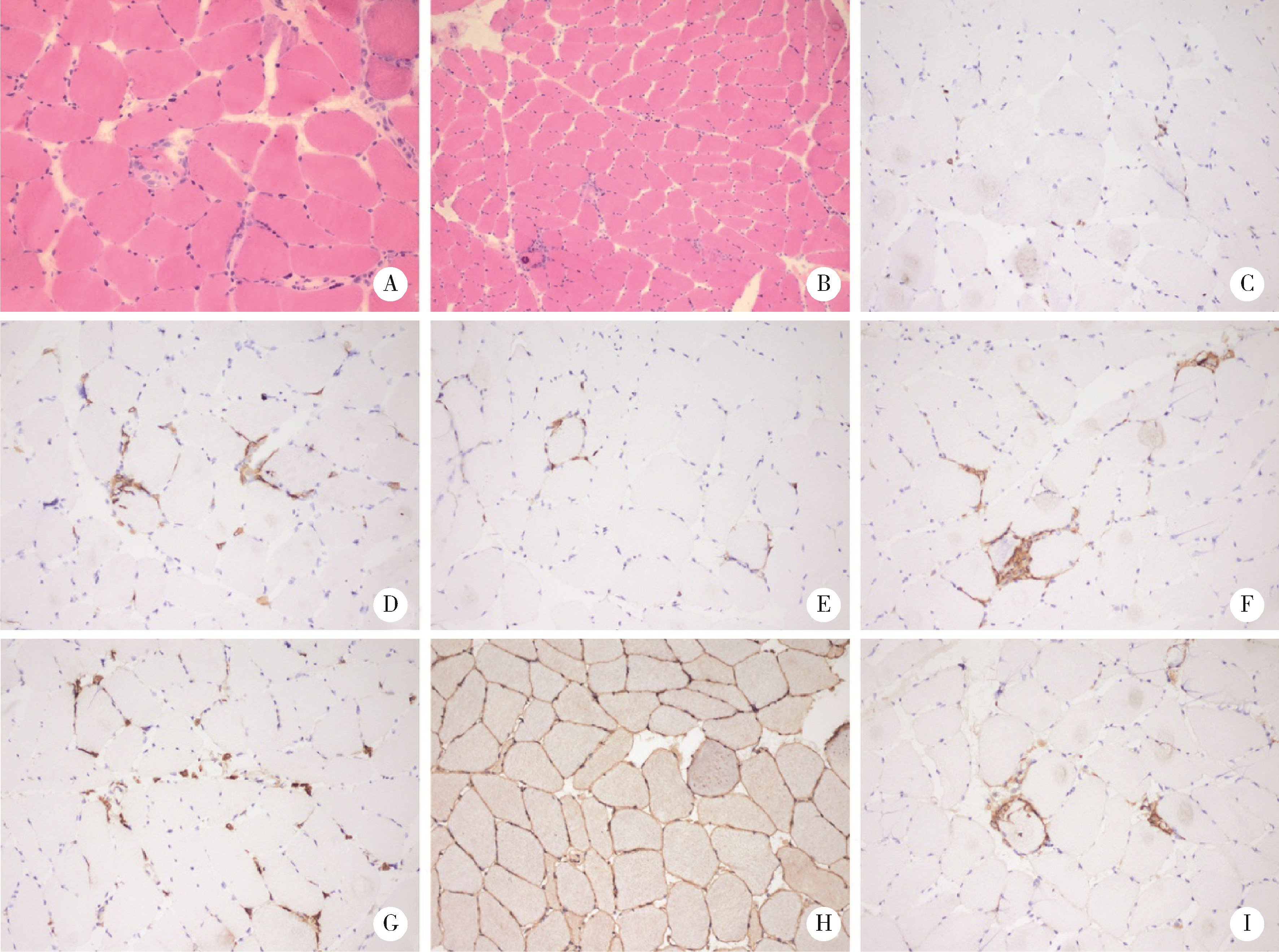
目的: 分析泌尿肿瘤免疫检查点抑制剂(immune checkpoint inhibitor, ICI)相关性肌炎的临床特征及治疗转归。方法: 选择2018年3月—2022年3月北京大学第一医院泌尿外科诊治的8例泌尿肿瘤ICI治疗后免疫相关性肌炎患者的临床资料进行回顾性分析,对人口学特征、用药方案、临床症状、实验室指标、肌电图检查、病理表现、治疗转归等信息进行分析。结果: 8例患者包含女性2例、男性6例,中位年龄68岁,均因泌尿肿瘤接受ICI治疗,包括2例上尿路尿路上皮癌(upper tract urothelial carcinoma,UTUC)、3例肾细胞癌(renal cell carcinoma,RCC)和3例膀胱癌(bladder cancer, BCa)。首次ICI治疗至发现免疫相关性肌炎的中位时间为39.5 d,中位疗程为2个疗程。主要症状为肌肉酸痛乏力,5例伴眼睑下垂,3例继发横纹肌溶解,5例合并心肌炎,1例合并重症肌无力,1例合并肠炎。发现合并免疫相关性心肌炎的患者首次接受ICI治疗至肌炎起病的间隔时间更短(P=0.042)。8例患者均有转氨酶及肌酶谱指标显著升高,5例患者出现自身抗体阳性。3例患者完善了肌肉活检,表现出典型的骨骼肌炎性肌病样病理改变,伴CD3+、CD4+、CD8+、CD20+淋巴细胞和CD68+巨噬细胞浸润。诊断免疫相关性肌炎后8例患者均立即停用ICI治疗,使用甲泼尼龙单独或合并丙种球蛋白静脉注射后病情均好转。结论: ICI治疗后免疫相关性肌炎是具有独特临床及病理特征的免疫相关不良反应(immune-related adverse events, irAEs),常见合并心血管不良反应,立即停用ICI并开始糖皮质激素治疗可以及时改善患者病情。
中图分类号:
- R737
| 1 |
Arnaud-Coffin P , Maillet D , Gan HK , et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors[J]. Int J Cancer, 2019, 145 (3): 639- 648.
doi: 10.1002/ijc.32132 |
| 2 |
Naidoo J , Page DB , Li BT , et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies[J]. Ann Oncol, 2015, 26 (12): 2375- 2391.
doi: 10.1093/annonc/mdv383 |
| 3 | 中国临床肿瘤学会指南工作委员会. 免疫检查点抑制剂相关的毒性管理指南[M]. 2019版 北京: 人民卫生出版社, 2019: 4. |
| 4 | NCCN Guidelines Version 1. 2022 management of immunotherapy-related toxicities[EB/OL]. [2022-02-28]. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. |
| 5 |
Vaddepally RK , Kharel P , Pandey R , et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence[J]. Cancers (Basel), 2020, 12 (3): 738.
doi: 10.3390/cancers12030738 |
| 6 |
Ernstoff MS , Gandhi S , Pandey M , et al. Challenges faced when identifying patients for combination immunotherapy[J]. Future Oncol, 2017, 13 (18): 1607- 1618.
doi: 10.2217/fon-2017-0218 |
| 7 |
Allenbach Y , Anquetil C , Manouchehri A , et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities[J]. Autoimmun Rev, 2020, 19 (8): 102586.
doi: 10.1016/j.autrev.2020.102586 |
| 8 |
Aldrich J , Pundole X , Tummala S , et al. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors[J]. Arthritis Rheumatol, 2021, 73 (5): 866- 874.
doi: 10.1002/art.41604 |
| 9 | Moslehi JJ , Salem JE , Sosman JA , et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis[J]. Lancet, 2018, 391 (10124): 933. |
| 10 |
Matas-García A , Milisenda JC , Selva-O'Callaghan A , et al. Emerging PD-1 and PD-1L inhibitors-associated myopathy with a characteristic histopathological pattern[J]. Autoimmun Rev, 2020, 19 (2): 102455.
doi: 10.1016/j.autrev.2019.102455 |
| 11 |
Liewluck T , Kao JC , Mauermann ML . PD-1 Inhibitor-associated myopathies: Emerging immune-mediated myopathies[J]. J Immunother, 2018, 41 (4): 208- 211.
doi: 10.1097/CJI.0000000000000196 |
| 12 |
Johnson DB , Balko JM , Compton ML , et al. Fulminant myocarditis with combination immune checkpoint blockade[J]. N Engl J Med, 2016, 375 (18): 1749- 1755.
doi: 10.1056/NEJMoa1609214 |
| 13 |
Mahmood SS , Fradley MG , Cohen JV , et al. Myocarditis in patients treated with immune checkpoint inhibitors[J]. J Am Coll Cardiol, 2018, 71 (16): 1755- 1764.
doi: 10.1016/j.jacc.2018.02.037 |
| 14 |
Moreira A , Loquai C , Pföhler C , et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors[J]. Eur J Cancer, 2019, 106, 12- 23.
doi: 10.1016/j.ejca.2018.09.033 |
| 15 |
Wang DY , Salem JE , Cohen JV , et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis[J]. JAMA Oncol, 2018, 4 (12): 1721- 1728.
doi: 10.1001/jamaoncol.2018.3923 |
| 16 |
Awadalla M , Mahmood SS , Groarke JD , et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis[J]. J Am Coll Cardiol, 2020, 75 (5): 467- 478.
doi: 10.1016/j.jacc.2019.11.049 |
| 17 |
Dolladille C , Ederhy S , Allouche S , et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors[J]. J Immunother Cancer, 2020, 8 (1): e000261.
doi: 10.1136/jitc-2019-000261 |
| 18 |
Salem JE , Manouchehri A , Moey M , et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study[J]. Lancet Oncol, 2018, 19 (12): 1579- 1589.
doi: 10.1016/S1470-2045(18)30608-9 |
| 19 |
Weill A , Delyon J , Descamps V , et al. Treatment strategies and safety of rechallenge in the setting of immune checkpoint inhibitors-related myositis: a national multicentre study[J]. Rheumatology (Oxford), 2021, 60 (12): 5753- 5764.
doi: 10.1093/rheumatology/keab249 |
| 20 |
Pollack MH , Betof A , Dearden H , et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma[J]. Ann Oncol, 2018, 29 (1): 250- 255.
doi: 10.1093/annonc/mdx642 |
| [1] | 刘家骏, 刘国康, 朱玉虎. 免疫相关性重症肺炎1例[J]. 北京大学学报(医学版), 2024, 56(5): 932-937. |
| [2] | 邢晓燕,张筠肖,朱冯赟智,王一帆,周新尧,李玉慧. 皮肌炎合并巨噬细胞活化综合征5例[J]. 北京大学学报(医学版), 2022, 54(6): 1214-1218. |
| [3] | 秦彩朋,宋宇轩,丁梦婷,王飞,林佳兴,杨文博,杜依青,李清,刘士军,徐涛. 肾癌免疫治疗疗效评估突变预测模型的建立[J]. 北京大学学报(医学版), 2022, 54(4): 663-668. |
| [4] | 刘圣杰,侯惠民,吕政通,丁鑫,王璐,张磊,刘明. 双极雄激素序贯免疫检查点抑制剂治疗转移性去势抵抗性前列腺癌4例[J]. 北京大学学报(医学版), 2022, 54(4): 766-769. |
| [5] | 顾阳春,刘颖,谢超,曹宝山. 程序性死亡蛋白-1抑制剂治疗晚期肺癌出现垂体免疫不良反应3例[J]. 北京大学学报(医学版), 2022, 54(2): 369-375. |
| [6] | 张朴丽,杨红霞,张立宁,葛勇鹏,彭清林,王国春,卢昕. 血清YKL-40在诊断抗黑色素瘤分化相关基因5阳性皮肌炎合并严重肺损伤中的价值[J]. 北京大学学报(医学版), 2021, 53(6): 1055-1060. |
| [7] | 罗澜,邢晓燕,肖云抒,陈珂彦,朱冯赟智,张学武,李玉慧. 抗合成酶综合征合并心脏受累患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1078-1082. |
| [8] | 肖云抒,朱冯赟智,罗澜,邢晓燕,李玉慧,张学武,沈丹华. 88例重叠肌炎的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1088-1093. |
| [9] | 伊文霞,魏翠洁,吴晔,包新华,熊晖,常杏芝. 长疗程利妥昔单抗治疗难治性幼年型特发性炎症性肌病3例[J]. 北京大学学报(医学版), 2021, 53(6): 1191-1195. |
| [10] | 吴燕芳,高飞,林滇恬,陈志涵,林禾. 托法替布联合治疗抗MDA5抗体阳性的无肌病皮肌炎并发快速进展型间质性肺病5例临床分析[J]. 北京大学学报(医学版), 2021, 53(5): 1012-1016. |
| [11] | 朱冯赟智,邢晓燕,汤晓菲,李依敏,邵苗,张学武,李玉慧,孙晓麟,何菁. 肌炎合并血栓栓塞患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2020, 52(6): 995-1000. |
| [12] | 郑艺明,郝洪军,刘怡琳,郭晶,赵亚雯,张巍,袁云. Ro52抗体与其他肌炎抗体共阳性的相关性研究[J]. 北京大学学报(医学版), 2020, 52(6): 1088-1092. |
| [13] | 甘雨舟,李玉慧,张丽华,马琳,何文雯,金月波,安媛,栗占国,叶华. 临床无肌病性皮肌炎与皮肌炎临床及免疫学特征比较[J]. 北京大学学报(医学版), 2020, 52(6): 1001-1008. |
| [14] | 王皓,姜树坤,彭冉,黄毅,王明清,王俊杰,刘承,张帆,马潞林. 个体化尿量控制提高泌尿肿瘤放疗期间膀胱稳定性[J]. 北京大学学报(医学版), 2020, 52(4): 688-691. |
| [15] | 徐婧,徐静,李鹤,唐杰,舒建龙,张婧,石连杰,李胜光. 皮肌炎合并IgA血管炎1例[J]. 北京大学学报(医学版), 2019, 51(6): 1173-1177. |
|
||