北京大学学报(医学版) ›› 2019, Vol. 51 ›› Issue (1): 35-42. doi: 10.19723/j.issn.1671-167X.2019.01.007
原发性腮腺淋巴瘤的临床病理特点及预后分析
- 1. 北京大学口腔医学院·口腔医院,口腔颌面外科,北京 100081
2. 北京大学口腔医学院·口腔医院, 病理科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Clinicopathological features and possible prognostic factors in parotid lymphomas
Qian SU1,Xin PENG1,Chuan-xiang ZHOU2,△( ),Guang-yan YU1,△(
),Guang-yan YU1,△( )
)
- 1. Department of Oral and Maxillofacial Surgery, Beijing 100081, China
2. Department of Oral Pathology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
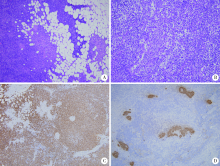
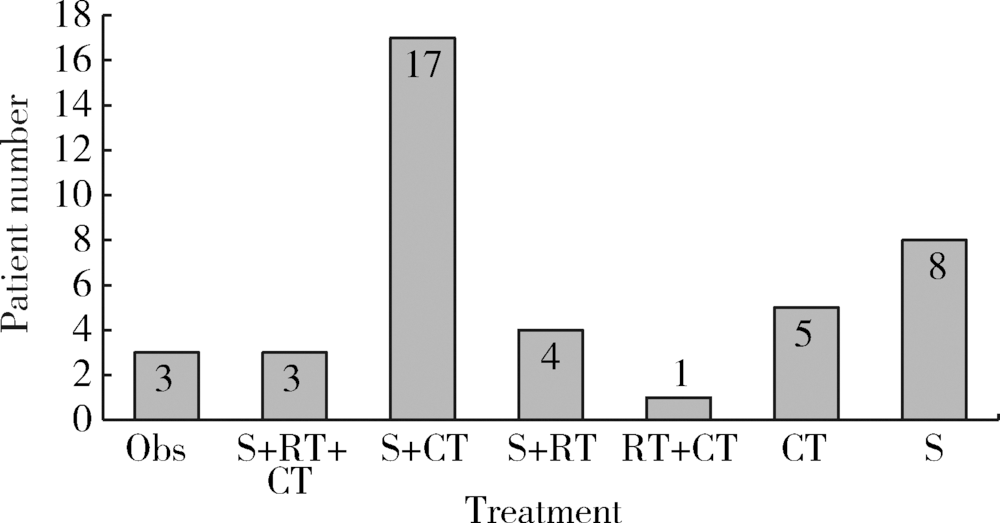
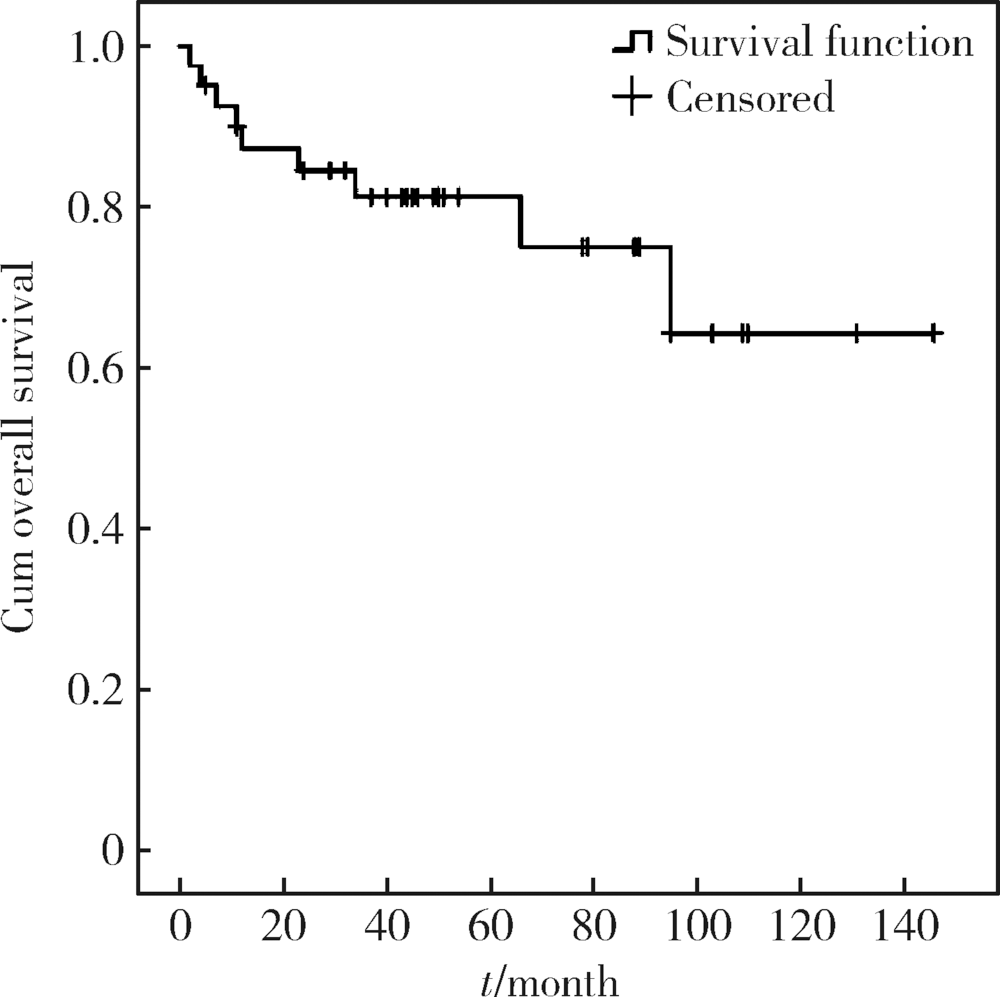
目的: 探讨原发性腮腺淋巴瘤的临床病理特点及影响其预后的因素。方法: 收集2006—2016年于北京大学口腔医院口腔颌面外科就诊并诊断为原发性腮腺淋巴瘤患者的相关临床资料,结合复查随访资料,回顾性总结其临床病理特点及患者预后,分析影响预后的相关因素。结果:研究共纳入41例患者,中位年龄57岁(8个月至91岁),男 ∶女=1 ∶2.15,女性多见。40例(97.1%)为非霍奇金淋巴瘤(non-Hodgkin lymphoma,NHL),其中结外边缘区黏膜相关淋巴组织淋巴瘤(extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue,MALT)15例,弥漫性大B细胞淋巴瘤(diffuse large B cell lymphoma,DLBCL)14例,滤泡型淋巴瘤(follicular lymphoma,FL)4例,其他类型少见,霍奇金淋巴瘤(Hodgkin lymphoma,HL)仅占1例。Ann Arbor ⅠE~ⅡE期37例(90.2%),ⅢE~ⅣE期4例(9.8%)。7例(17.1%)淋巴瘤由干燥综合征恶变而来,均为MALT淋巴瘤。平均病期为20.7月,78%的患者以腮腺区缓慢生长的无痛性肿块为主要症状。治疗方法包括局部治疗及系统治疗,不同治疗方案均可取得较好疗效。全部患者均经5~149个月随访,其中9例(21.9%)死亡,2年总生存率为84.5%,5年总生存率为81.3%。单因素分析显示有无肿物加速生长史(P=0.005)和肿物有无包膜(P=0.011)为总生存率的影响因素,多因素分析显示肿物有无包膜(P=0.041)影响患者的总生存期。结论:腮腺淋巴瘤绝大多数为B细胞型NHL,MALT淋巴瘤与DLBCL最为常见,大部分患者表现为缓慢生长的腮腺区局限性肿块,临床上需与腮腺良性肿瘤相鉴别。干燥综合征与MALT淋巴瘤的发病密切相关。原发性腮腺淋巴瘤患者预后比其他恶性肿瘤相对较好,肿物无包膜提示预后较差。
中图分类号:
- R733.41
| [1] | 陈万青, 郑荣寿, 张思维 , 等. 2013年中国恶性肿瘤发病和死亡分析[J]. 中国肿瘤, 2017,26(1):1-7. |
| [2] |
李小秋, 李甘地, 高子芬 , 等. 中国淋巴瘤亚型分布:国内多中心性病例10 002例分析[J]. 诊断学理论与实践, 2012,11(2):111-115.
doi: 10.3969/j.issn.1671-2870.2012.02.006 |
| [3] |
Urquhart A, Berg R . Hodgkin’s and non-Hodgkin’s lymphoma of the head and neck[J]. Laryngoscope, 2001,111(9):1565-1569.
doi: 10.1097/00005537-200109000-00013 pmid: 11568605 |
| [4] |
Etemad-Moghadam S, Tirgary F, Keshavarz S , et al. Head and neck non-Hodgkin’s lymphoma: a 20-year demographic study of 381 cases[J]. Int J Oral Max Surg, 2010,39(9):869-872.
doi: 10.1016/j.ijom.2010.03.029 pmid: 20538427 |
| [5] | Swerdlow SH, Campo E, Harris NL , et al. WHO classification of tumours of haematopoietic and lymphoid tissues[M]. 4th ed. Iyon: International Agency for Research on Cancer, 2017. |
| [6] |
Skarin AT, Dorfman DM . Non-Hodgkin’s lymphomas: Current classification and management[J]. CA Cancer J Clin, 1997,47(6):351-372.
doi: 10.3322/canjclin.47.6.351 pmid: 9371057 |
| [7] |
Gleeson MJ, Bennett MH, Cawson RA . Lymphomas of salivary glands[J]. Cancer, 1986,58(3):699-704.
doi: 10.1002/(ISSN)1097-0142 |
| [8] | El-Naggar AK, Chan JKC, Grandis JR , et al. WHO classification of head and neck tumors[M]. 4th ed. Lyon: International Agency for Research on Cancer, 2017: 276-280. |
| [9] |
Feinstein AJ, Ciarleglio MM, Cong X , et al. Parotid gland lymphoma: prognostic analysis of 2140 patients[J]. Laryngoscope, 2013,123(5):1199-1203.
doi: 10.1002/lary.23901 pmid: 23576299 |
| [10] |
Vazquez A, Khan MN, Sanghvi S , et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the salivary glands: a population-based study from 1994 to 2009[J]. Head Neck, 2015,37(1):18-22.
doi: 10.1002/hed.23543 pmid: 24733777 |
| [11] |
Isaacson P, Wright DH . Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma[J]. Cancer, 1983,52(8):1410-1416.
doi: 10.1002/(ISSN)1097-0142 |
| [12] | 徐鹏程, 周心一, 周晨 , 等. 腮腺黏膜相关淋巴组织淋巴瘤的临床特点分析[J]. 临床耳鼻咽喉头颈外科杂志, 2017(1):61-64. |
| [13] |
Ambrosetti A, Zanotti R, Pattaro C , et al. Most cases of primary salivary mucosa-associated lymphoid tissue lymphoma are associa-ted either with Sjögren syndrome or hepatitis C virus infection[J]. Br J Haematol, 2004,126(1):43-49.
doi: 10.1111/j.1365-2141.2004.04993.x pmid: 15198730 |
| [14] |
Dong L, Chen Y, Masaki Y , et al. Possible mechanisms of lymphoma development in Sjögren’s syndrome[J]. Curr Immunol Rev, 2013,9(1):13-22.
doi: 10.2174/1573395511309010003 |
| [15] |
Zhang W, Feng S, Yan S , et al. Incidence of malignancy in primary Sjögren’s syndrome in a Chinese cohort[J]. Rheumatology (Oxford), 2010,49(3):571-577.
doi: 10.1093/rheumatology/kep404 pmid: 20040528 |
| [16] |
Theander E, Henriksson G, Ljungberg O , et al. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors[J]. Ann Rheum Dis, 2006,65(6):796-803.
doi: 10.1136/ard.2005.041186 pmid: 1798187 |
| [17] |
卢松鹤, 闫志敏, 魏明洁 , 等. 结节型舍格伦综合征与恶性淋巴瘤关系初探[J]. 中华口腔医学杂志, 2012,47(4):208-213.
doi: 10.3760/cma.j.issn.1002-0098.2012.04.004 |
| [18] |
Peveling-Oberhag J, Arcaini L, Bankov K , et al. The anti-lymphoma activity of antiviral therapy in HCV-associated B-cell non-Hodgkin lymphomas: a meta-analysis[J]. J Viral Hepat, 2016,23(7):536-544.
doi: 10.1111/jvh.12518 pmid: 26924533 |
| [19] |
Arcaini L, Rossi D, Lucioni M , et al. The NOTCH pathway is recurrently mutated in diffuse large B-cell lymphoma associated with hepatitis C virus infection[J]. Haematologica, 2015,100(2):246-252.
doi: 10.3324/haematol.2014.116855 pmid: 25381127 |
| [20] |
Brito-Zerón P, Gheitasi H, Retamozo S , et al. How hepatitis C virus modifies the immunological profile of Sjögren syndrome: analysis of 783 patients[J]. Arthritis Res, 2015,17(1):1-9.
doi: 10.1186/s13075-014-0514-0 pmid: 4318544 |
| [21] |
Raderer M, Kiesewetter B, Ferreri AJ . Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma)[J]. CA Cancer J Clin, 2016,66(2):153-171.
doi: 10.3322/caac.21330 pmid: 26773441 |
| [22] |
Swerdlow SH, Campo E, Pileri SA , et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016,127(20):2375-2390.
doi: 10.1182/blood-2016-01-643569 |
| [23] |
Thielker J, Grosheva M, Ihrler S , et al. Contemporary management of benign and malignant parotid tumors[J]. Front Surg, 2018,39(5):1-17.
doi: 10.3389/fsurg.2018.00039 |
| [24] |
Gudmundsson JK, Ajan A, Abtahi J . The accuracy of fine-needle aspiration cytology for diagnosis of parotid gland masses: a clinicopathological study of 114 patients[J]. J Appl Oral Sci, 2016,24(6):561-567.
doi: 10.1590/1678-775720160214 pmid: 5161254 |
| [25] |
Orita Y, Sato Y, Kimura N , et al. Characteristic ultrasound features of mucosa-associated lymphoid tissue lymphoma of the salivary and thyroid gland[J]. Acta Otolaryngol, 2014,134(1):93-99.
doi: 10.3109/00016489.2013.831994 pmid: 24256049 |
| [26] |
高润涛, 耿雪霏 . 8例腮腺恶性淋巴瘤诊疗体会[J]. 口腔医学研究, 2017,33(8):870-873.
doi: 10.13701/j.cnki.kqyxyj.2017.08.017 |
| [27] |
Olszewski AJ, Desai A . Radiation therapy administration and survival in stage Ⅰ/Ⅱ extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue[J]. Int J Radiat Oncol Biol Phys, 2014,88(3):642-649.
doi: 10.1016/j.ijrobp.2013.11.225 pmid: 24411627 |
| [28] |
Jackson AE, Mian M, Kalpadakis C , et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the salivary glands: a multicenter, international experience of 248 patients (IELSG 41)[J]. Oncologist, 2015,20(10):1149-1153.
doi: 10.1634/theoncologist.2015-0180 pmid: 26268740 |
| [29] |
Salles G, Barrett M, Foa R , et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience[J]. Adv Ther, 2017,34(10):2232-2273.
doi: 10.1007/s12325-017-0612-x pmid: 5656728 |
| [30] |
傅志英, 朱军, 宋玉琴 , 等. 525例弥漫大B细胞淋巴瘤预后影响因素分析[J]. 北京大学学报(医学版), 2014,46(3):405-411.
doi: 10.3969/j.issn.1671-167X.2014.03.013 |
| [31] | 杨小芸, 沈丽达, 龙庭凤 , 等. 1 326例非霍奇金淋巴瘤临床病理特点分析[J]. 中华肿瘤防治杂志, 2016,23(9):605-609. |
| [1] | 杨玉淑, 齐晅, 丁萌, 王炜, 郭惠芳, 高丽霞. 抗唾液腺蛋白1抗体联合抗腮腺分泌蛋白抗体对干燥综合征的诊断价值[J]. 北京大学学报(医学版), 2024, 56(5): 845-852. |
| [2] | 欧俊永,倪坤明,马潞林,王国良,颜野,杨斌,李庚午,宋昊东,陆敏,叶剑飞,张树栋. 肌层浸润性膀胱癌合并中高危前列腺癌患者的预后因素[J]. 北京大学学报(医学版), 2024, 56(4): 582-588. |
| [3] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [4] | 虞乐,邓绍晖,张帆,颜野,叶剑飞,张树栋. 具有低度恶性潜能的多房囊性肾肿瘤的临床病理特征及预后[J]. 北京大学学报(医学版), 2024, 56(4): 661-666. |
| [5] | 周泽臻,邓绍晖,颜野,张帆,郝一昌,葛力源,张洪宪,王国良,张树栋. 非转移性T3a肾细胞癌患者3年肿瘤特异性生存期预测[J]. 北京大学学报(医学版), 2024, 56(4): 673-679. |
| [6] | 方杨毅,李强,黄志高,陆敏,洪锴,张树栋. 睾丸鞘膜高分化乳头状间皮肿瘤1例[J]. 北京大学学报(医学版), 2024, 56(4): 741-744. |
| [7] | 曾媛媛,谢云,陈道南,王瑞兰. 脓毒症患者发生正常甲状腺性病态综合征的相关因素[J]. 北京大学学报(医学版), 2024, 56(3): 526-532. |
| [8] | 苏俊琪,王晓颖,孙志强. 舌鳞状细胞癌根治性切除术后患者预后预测列线图的构建与验证[J]. 北京大学学报(医学版), 2024, 56(1): 120-130. |
| [9] | 李建斌,吕梦娜,池强,彭一琳,刘鹏程,吴锐. 干燥综合征患者发生重症新型冠状病毒肺炎的早期预测[J]. 北京大学学报(医学版), 2023, 55(6): 1007-1012. |
| [10] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [11] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [12] | 卢汉,张建运,杨榕,徐乐,李庆祥,郭玉兴,郭传瑸. 下颌牙龈鳞状细胞癌患者预后的影响因素[J]. 北京大学学报(医学版), 2023, 55(4): 702-707. |
| [13] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
| [14] | 朱晓娟,张虹,张爽,李东,李鑫,徐玲,李挺. 人表皮生长因子受体2低表达乳腺癌的临床病理学特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 243-253. |
| [15] | 赖玉梅,李忠武,李欢,吴艳,时云飞,周立新,楼雨彤,崔传亮. 68例肛管直肠黏膜黑色素瘤临床病理特征及预后[J]. 北京大学学报(医学版), 2023, 55(2): 262-269. |
|
||