北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (1): 30-37. doi: 10.19723/j.issn.1671-167X.2023.01.005
种植体黏膜下微生物在健康种植体和种植体周炎中的构成与差异:一项横断面研究
- 北京大学口腔医学院·口腔医院牙周科,国家口腔医学中心,国家口腔疾病临床医学研究中心,口腔生物材料和数字诊疗装备国家工程研究中心,北京 100081
Profiles and differences of submucosal microbial in peri-implantitis and health implants: A cross-sectional study
Fei SUN,Jian LIU,Si-qi LI,Yi-ping WEI,Wen-jie HU*( ),Cui WANG*(
),Cui WANG*( )
)
- Department of Periodontology, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
摘要:
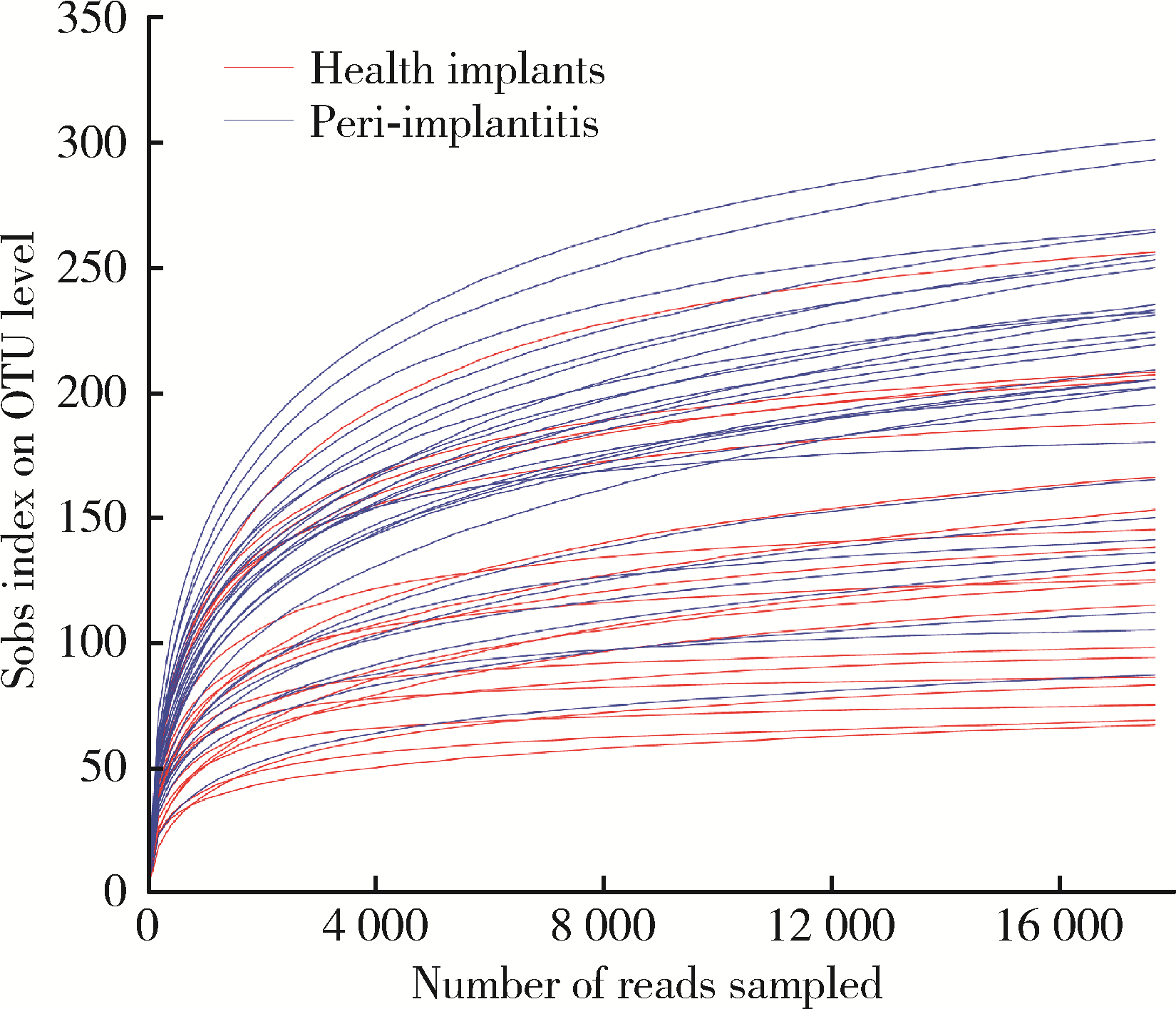
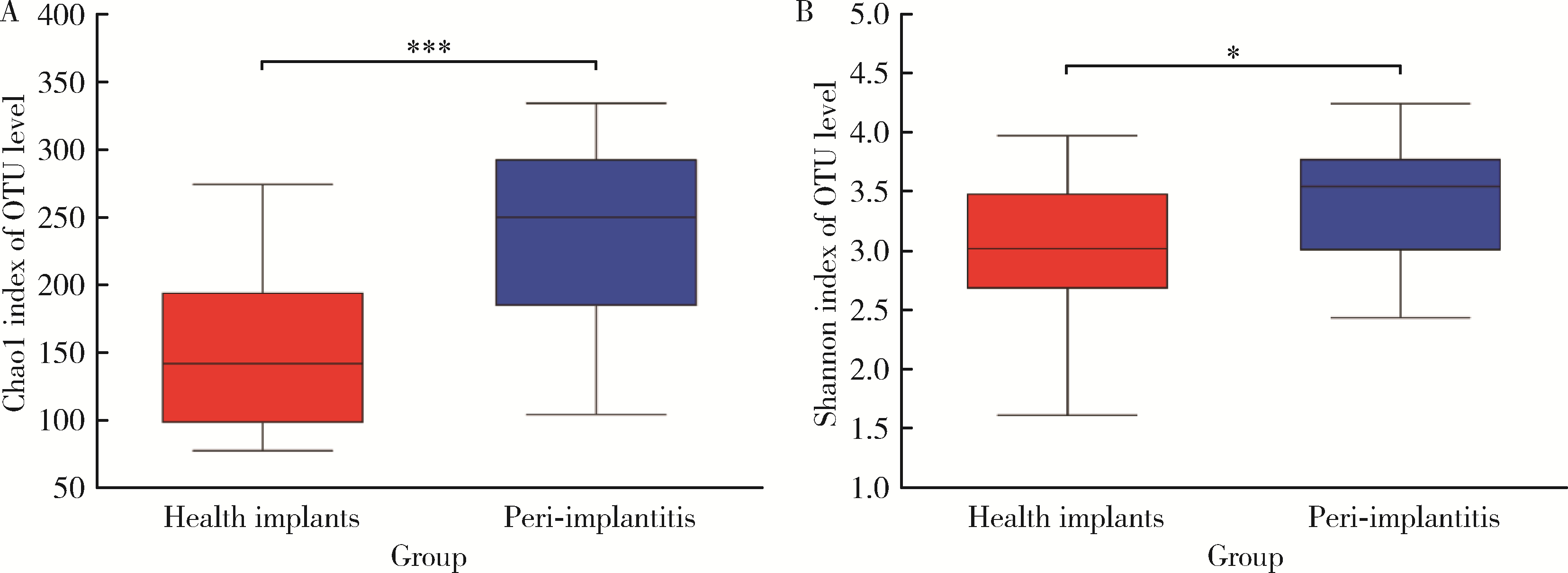
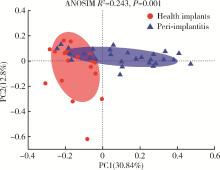
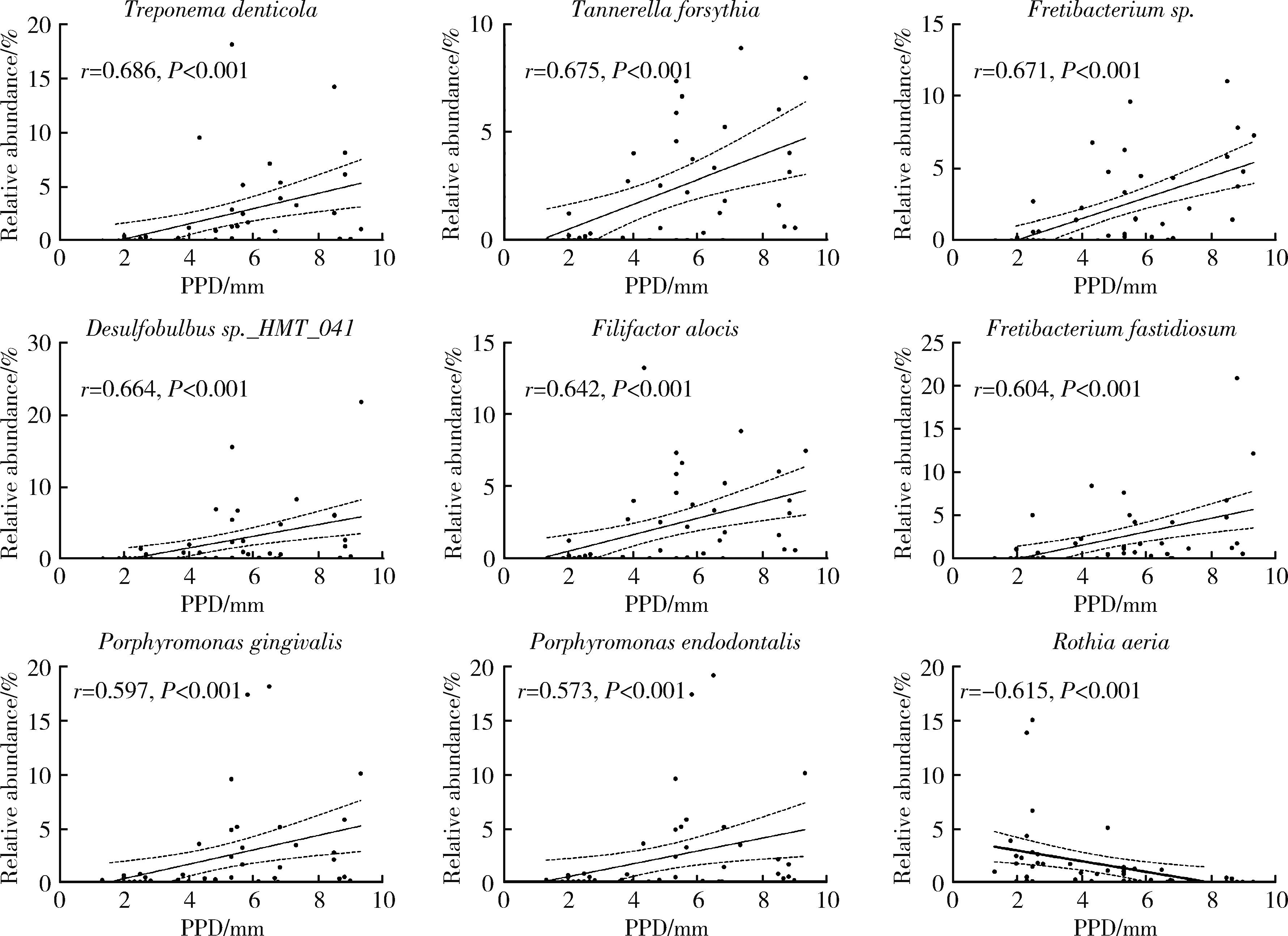
目的: 探索种植体黏膜下微生物在健康种植体和种植体周炎中的构成与差异,并分析与临床指标存在相关性的菌种,为种植体周炎的病因学研究提供参考。方法: 采用横断面研究,共纳入49例患者,20例为健康种植体,29例为种植体周炎,共采集49份黏膜下微生物样本进行16S核糖体RNA(16S ribosomal RNA, 16S rRNA)基因高通量测序。对两组样本的多样性、菌群构成和差异物种进行分析和比较,采用Spearman相关性分析评价菌种与探诊深度(probing pocket depth, PPD)之间的相关性。结果: 健康组的α多样性显著低于种植体周炎组[Chao1指数:236.85±66.13 vs. 150.54±57.43,P < 0.001;Shannon指数:3.42±0.48 vs. 3.02±0.65,P=0.032]。主成分分析显示,两组样本的群落结构差异有统计学意义[相似性分析(analysis of similarities, ANOSIM),R2=0.243,P=0.001]。与健康种植体相比,种植体周炎黏膜下菌斑中的牙周致病菌显著增加,包括红色复合体成员[牙龈卟啉单胞菌(Porphyromonas gingivalis)、福赛斯坦纳菌(Tannerella forsythia)、齿垢密螺旋体(Treponema denticola)]、部分橙色复合体成员[中间普氏菌(Precotella intermedia)、缠结优杆菌(Eubacterium nodatum)和微小微单胞菌(Parvimonas micra)]以及一些新型牙周致病菌,如龈沟产线菌(Fillifactor alocis)、苛求依赖杆菌(Fretibacterium fastidiosum)、牙髓卟啉单胞菌(Porphyromonas endodontalis)和Desulfobulbus sp._HMT_041。相关性分析显示,种植体黏膜下微生物中,齿垢密螺旋体(r=0.686, P < 0.001)、福赛斯坦纳菌(r=0.675, P < 0.001)、Fretibacterium sp. (r=0.671, P < 0.001)、Desulfobulbus sp._HMT_041 (r=0.664, P < 0.001)、龈沟产线菌(r=0.642, P < 0.001)、苛求依赖杆菌(r=0.604, P < 0.001)、牙龈卟啉单胞菌(r=0.597, P < 0.001)、牙髓卟啉单胞菌(r=0.573, P < 0.001)的丰度与种植体周PPD呈显著正向相关;空间罗氏菌(Rothia aeria)(r=-0.615, P < 0.001)的丰度与PPD呈显著负相关。结论: 种植体黏膜下微生物在健康种植体和种植体周炎中的群落构成存在明显不同,红色复合体、部分橙色复合体成员以及一些新型牙周致病菌与种植体周炎的发生密切相关;与健康种植体相比,种植体周炎的黏膜下微生物群落具有高物种丰富度及多样性的特点。
中图分类号:
- R781.4
| 1 |
Howe MS , Keys W , Richards D . Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis[J]. J Dent, 2019, 84, 9- 21.
doi: 10.1016/j.jdent.2019.03.008 |
| 2 | Schwarz F , Derks J , Monje A , et al. Peri-implantitis[J]. J Periodontol, 2018, 89 (Suppl 1): S267- S290. |
| 3 |
Kordbacheh Changi K , Finkelstein J , Papapanou PN . Peri-implan-titis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school[J]. Clin Oral Implants Res, 2019, 30 (4): 306- 314.
doi: 10.1111/clr.13416 |
| 4 | Berglundh T , Armitage G , Araujo MG , et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions[J]. J Clin Periodontol, 2018, 45 (Suppl 20): S286- S291. |
| 5 | Renvert S , Persson GR , Pirih FQ , et al. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations[J]. J Periodontol, 2018, 89 (Suppl 1): S304- S312. |
| 6 | Araujo MG , Lindhe J . Peri-implant health[J]. J Clin Periodontol, 2018, 45 (Suppl 20): S230- S236. |
| 7 |
孙菲, 李思琪, 危伊萍, 等. 种植体周病非手术治疗中联合应用甘氨酸粉喷砂的临床效果评价[J]. 北京大学学报(医学版), 2022, 54 (1): 119- 125.
doi: 10.19723/j.issn.1671-167X.2022.01.019 |
| 8 |
de Waal YC , Eijsbouts HV , Winkel EG , et al. Microbial characteristics of peri-implantitis: A case-control study[J]. J Periodontol, 2017, 88 (2): 209- 217.
doi: 10.1902/jop.2016.160231 |
| 9 |
Paster BJ , Olsen I , Aas JA , et al. The breadth of bacterial diversity in the human periodontal pocket and other oral sites[J]. Periodontology 2000, 2006, 42 (1): 80- 87.
doi: 10.1111/j.1600-0757.2006.00174.x |
| 10 |
Renvert S , Roos-Jansaker AM , Lindahl C , et al. Infection at titanium implants with or without a clinical diagnosis of inflammation[J]. Clin Oral Implants Res, 2007, 18 (4): 509- 516.
doi: 10.1111/j.1600-0501.2007.01378.x |
| 11 |
Zheng H , Xu L , Wang Z , et al. Subgingival microbiome in patients with healthy and ailing dental implants[J]. Sci Rep, 2015, 5, 10948.
doi: 10.1038/srep10948 |
| 12 |
Sanz-Martin I , Doolittle-Hall J , Teles RP , et al. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing[J]. J Clin Periodontol, 2017, 44 (12): 1274- 1284.
doi: 10.1111/jcpe.12788 |
| 13 |
Nie J , Zhang Q , Zheng H , et al. Pyrosequencing of the subgingival microbiome in peri-implantitis after non-surgical mechanical debridement therapy[J]. J Periodontal Res, 2020, 55 (2): 238- 246.
doi: 10.1111/jre.12708 |
| 14 |
Socransky SS , Haffajee AD , Cugini MA , et al. Microbial complexes in subgingival plaque[J]. J Clin Periodontol, 1998, 25 (2): 134- 144.
doi: 10.1111/j.1600-051X.1998.tb02419.x |
| 15 |
Al-Ahmad A , Muzafferiy F , Anderson AC , et al. Shift of micro-bial composition of peri-implantitis-associated oral biofilm as revealed by 16S rRNA gene cloning[J]. J Med Microbiol, 2018, 67 (3): 332- 340.
doi: 10.1099/jmm.0.000682 |
| 16 |
da Silva ES , Feres M , Figueiredo LC , et al. Microbiological diversity of peri-implantitis biofilm by Sanger sequencing[J]. Clin Oral Implants Res, 2014, 25 (10): 1192- 1199.
doi: 10.1111/clr.12231 |
| 17 |
Oliveira RR , Fermiano D , Feres M , et al. Levels of candidate periodontal pathogens in subgingival biofilm[J]. J Dent Res, 2016, 95 (6): 711- 718.
doi: 10.1177/0022034516634619 |
| 18 |
Fu JH , Wang HL . Breaking the wave of peri-implantitis[J]. Periodontol 2000, 2020, 84 (1): 145- 160.
doi: 10.1111/prd.12335 |
| 19 |
Perez-Chaparro PJ , Duarte PM , Shibli JA , et al. The current weight of evidence of the microbiologic profile associated with peri-implantitis: A systematic review[J]. J Periodontol, 2016, 87 (11): 1295- 1304.
doi: 10.1902/jop.2016.160184 |
| 20 |
Kroger A , Hulsmann C , Fickl S , et al. The severity of human peri-implantitis lesions correlates with the level of submucosal microbial dysbiosis[J]. J Clin Periodontol, 2018, 45 (12): 1498- 1509.
doi: 10.1111/jcpe.13023 |
| 21 |
Galofré M , Palao D , Vicario M , et al. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial[J]. J Periodontal Res, 2018, 53 (3): 378- 390.
doi: 10.1111/jre.12523 |
| 22 |
Mombelli A . Maintenance therapy for teeth and implants[J]. Periodontol 2000, 2019, 79 (1): 190- 199.
doi: 10.1111/prd.12255 |
| 23 |
Berglundh T , Jepsen S , Stadlinger B , et al. Peri-implantitis and its prevention[J]. Clin Oral Implants Res, 2019, 30 (2): 150- 155.
doi: 10.1111/clr.13401 |
| 24 |
Mombelli A . Microbial colonization of the periodontal pocket and its significance for periodontal therapy[J]. Periodontol 2000, 2018, 76 (1): 85- 96.
doi: 10.1111/prd.12147 |
| [1] | 王聪伟,高敏,于尧,章文博,彭歆. 游离腓骨瓣修复下颌骨缺损术后义齿修复的临床分析[J]. 北京大学学报(医学版), 2024, 56(1): 66-73. |
| [2] | 丁茜,李文锦,孙丰博,谷景华,林元华,张磊. 表面处理对氧化钇和氧化镁稳定的氧化锆种植体晶相及断裂强度的影响[J]. 北京大学学报(医学版), 2023, 55(4): 721-728. |
| [3] | 孟令玮,李雪,高胜寒,李悦,曹瑞涛,张毅,潘韶霞. 三种方法建立大鼠种植体周炎模型的比较[J]. 北京大学学报(医学版), 2023, 55(1): 22-29. |
| [4] | 孙菲,李思琪,危伊萍,钟金晟,王翠,胡文杰. 种植体周病非手术治疗中联合应用甘氨酸粉喷砂的临床效果评价[J]. 北京大学学报(医学版), 2022, 54(1): 119-125. |
| [5] | 梁峰,吴敏节,邹立东. 后牙区单牙种植修复5年后的临床修复疗效观察[J]. 北京大学学报(医学版), 2021, 53(5): 970-976. |
| [6] | 释栋,曹婕,戴世爱,孟焕新. 植体周炎再生治疗短期疗效观察[J]. 北京大学学报(医学版), 2020, 52(1): 58-63. |
| [7] | 林春平,卢松鹤,朱浚鑫,胡洪成,岳兆国,唐志辉. 个性化根形种植体的螺纹形态对周围牙槽骨应力分布影响的三维有限元分析[J]. 北京大学学报(医学版), 2019, 51(6): 1130-1137. |
| [8] | 刘潇倩,陈秋雯,冯海兰,王兵,屈健,孙振,衡墨迪,潘韶霞. 无牙颌患者locator附着体种植覆盖义齿修复后口腔卫生维护的纵向研究[J]. 北京大学学报(医学版), 2019, 51(1): 136-144. |
| [9] | 吴敏节,邹立东,梁峰. 上前牙即刻种植即刻修复负载3年后软、硬组织变化的临床观察[J]. 北京大学学报(医学版), 2018, 50(4): 694-699. |
| [10] | 刘婧寅,陈飞,葛严军,魏菱,潘韶霞,冯海兰. 选择性激光熔化种植体对早期骨矿化沉积率的影响[J]. 北京大学学报(医学版), 2018, 50(1): 117-122. |
| [11] | 梁乃文,石磊,黄颖,邓旭亮. 不同形貌纯钛表面对人脐静脉内皮细胞生物学行为的影响[J]. 北京大学学报(医学版), 2017, 49(1): 43-048. |
| [12] | 李贝贝, 林野, 崔宏燕, 郝强, 胥加斌, 邸萍. 碳纤维增强“All-on-4”即刻修复体的临床评价[J]. 北京大学学报(医学版), 2016, 48(1): 133-137. |
| [13] | 崔宏燕, 邸萍, 李健慧, 林野, 刘蓉蓉. 电火花蚀刻技术在种植修复体制作中的应用[J]. 北京大学学报(医学版), 2015, 47(2): 336-339. |
| [14] | 韩劼, 陈智滨, 李玮, 孟焕新. 早期愈合阶段牙种植体周沟液骨代谢相关因子的检测和种植体稳定性共振频率分析[J]. 北京大学学报(医学版), 2015, 47(1): 37-41. |
| [15] | 吴敏节, 张相皞, 邹立东, 梁峰. 临时冠成型术后1年牙龈稳定性的临床观察[J]. 北京大学学报(医学版), 2014, 46(6): 954-957. |
|
||