北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (2): 283-291. doi: 10.19723/j.issn.1671-167X.2023.02.012
青少年特发性脊柱侧凸椎旁肌的病理特征
郑丹枫1,李君禹2,3,李佳曦4,张英爽5,钟延丰1,于淼2,3,*( )
)
- 1. 北京大学基础医学院病理学系/北京大学第三医院病理科, 北京 100191
2. 北京大学第三医院骨科, 北京 100191
3. 北京大学第三医院脊柱疾病研究北京重点实验室, 北京 100191
4. 北京大学基础医学院, 北京 100191
5. 北京大学第三医院神经内科, 北京 100191
Pathologic features of paraspinal muscle biopsies in patients with adolescent idiopathic scoliosis
Dan-feng ZHENG1,Jun-yu LI2,3,Jia-xi LI4,Ying-shuang ZHANG5,Yan-feng ZHONG1,Miao YU2,3,*( )
)
- 1. Department of Pathology, School of Basic Medical Sciences Peking University/Peking University Third Hospital, Beijing 100191, China
2. Departmant of Orthopaedics, Peking University Third Hospital, Beijing 100191, China
3. Beijing Key Laboratory of Spinal Disease Research, Peking University Third Hospital, Beijing 100191, China
4. School of Basic Medical Sciences, Peking University, Beijing 100191, China
5. Departmant of Neurology, Peking University Third Hospital, Beijing 100191, China
摘要:
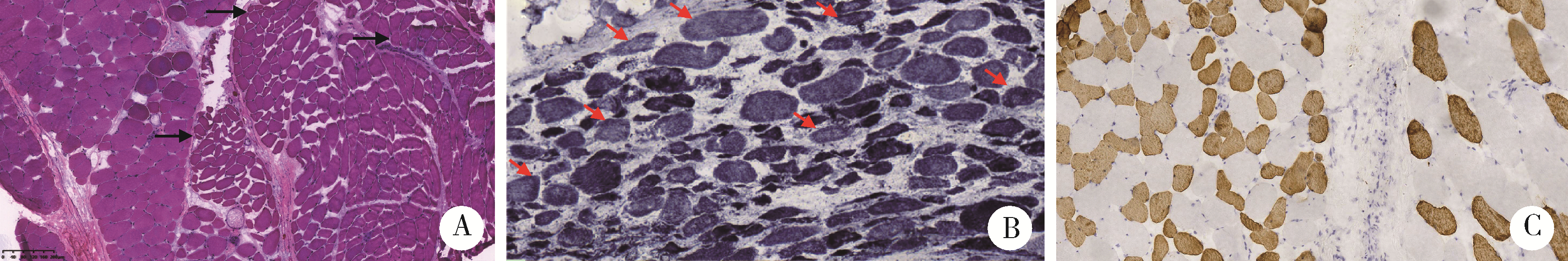
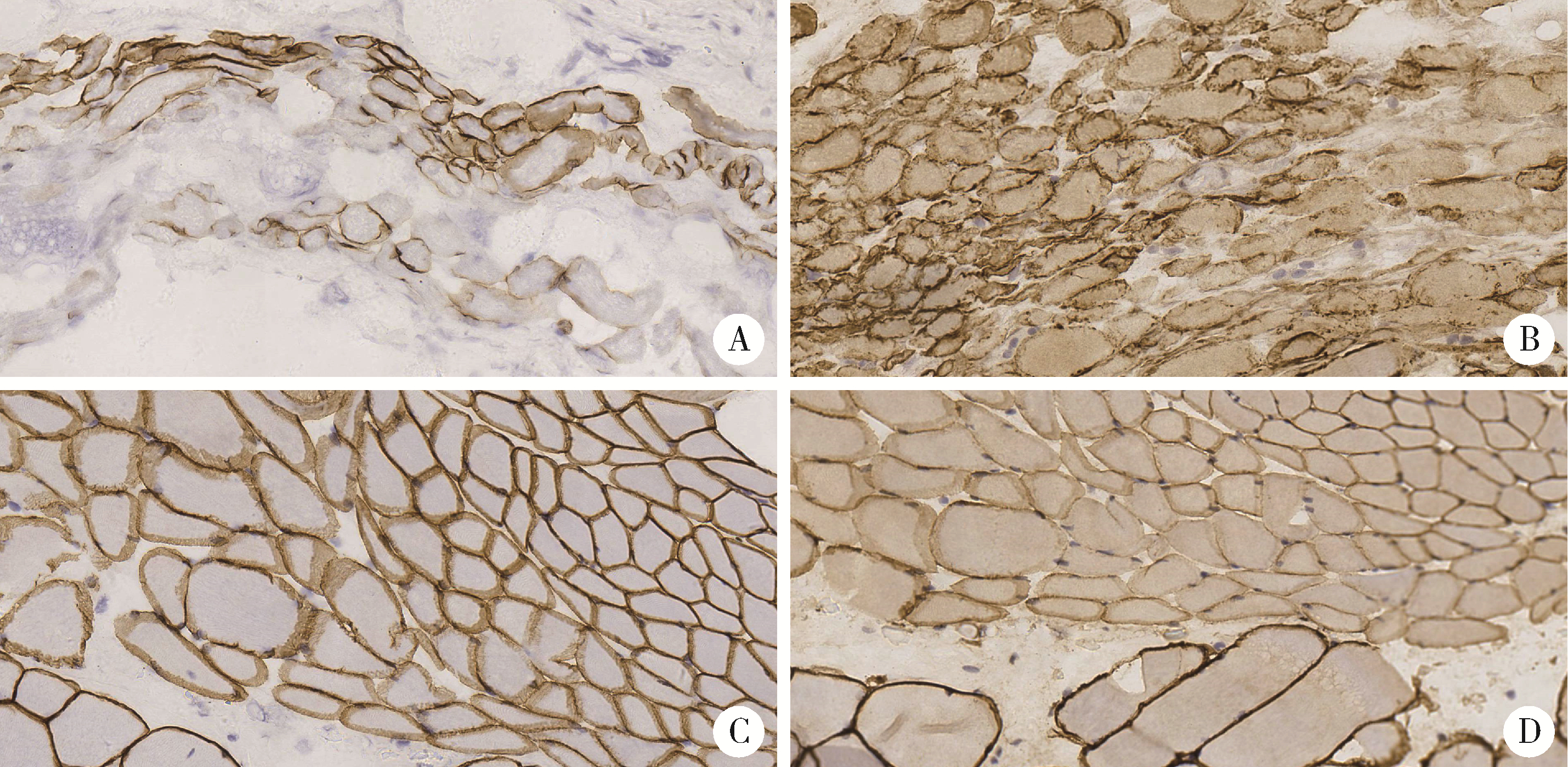
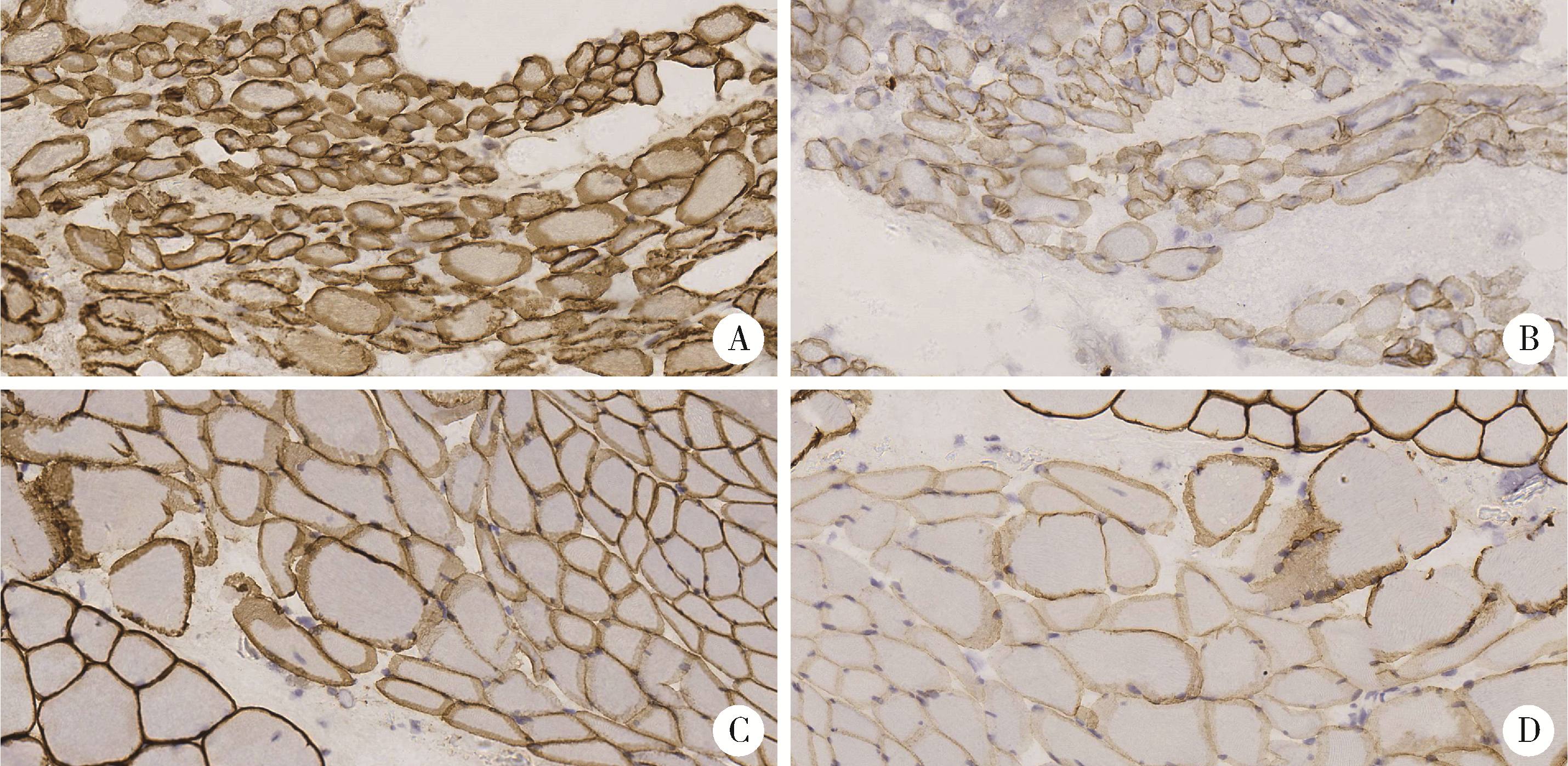
目的: 研究青少年特发性脊柱侧凸(adolescent idiopathic scoliosis, AIS)患者椎旁肌病理改变, 并进一步探讨抗肌萎缩蛋白(Dystrophin)在该病中的表达情况及其与病因的关系。方法: 收集2018年11月至2019年8月于北京大学第三医院诊断为AIS并接受后路侧弯矫形手术, 术中行凹侧顶椎椎旁肌活检患者18例。对肌肉活检组织进行规范化处理并切片进行常规苏木精-伊红(hematoxylin-eosin, HE)染色、多种组织化学染色以及抗肌萎缩蛋白各亚型、肌球蛋白、主要组织相容性复合体1(major histocompatibility complex 1, MHC-1)、CD4、CD8、CD20、CD68抗体的免疫组织化学染色及结果判读分析。此外, 根据Cobb角大小(≥55°或<55°)和Nash-Moe分类将活检标本分为不同组别, 进行相应病理改变的组间差异统计学比较。结果: 18例患者中, 重度AIS组(Cobb角≥55°)8例, 轻度AIS组(Cobb角 < 55°)10例, 两组患者椎旁肌均出现不同程度萎缩、变性, Dystrophin-3的免疫组织化学染色均不同程度缺失且表达模式异常。两组活检肌纤维的Dystrophin-2的免疫组织化学染色切片中的表达模式存在显著差异, 重度AIS组的Dystrophin-2表达异常或缺失更显著。根据Nash-Moe分型分组, 患者椎旁肌的Dystrophin-2表达模式也有显著差异, 表现为侧凸程度越重, Dystrophin-2表达异常越显著。此外, 椎旁肌肉组织的肌肉和肌腱中存在CD4+细胞和CD8+细胞的浸润, 而CD20+细胞为阴性。全部患者椎旁肌均有部分肌纤维呈MHC-1肌膜阳性表达。结论: AIS患者椎旁肌活检的组织学具有相似的特征性改变, 抗肌萎缩蛋白的表达不同程度降低或缺失, 并且与脊柱侧凸程度有一定相关性, 提示抗肌萎缩蛋白功能障碍在脊柱侧凸的发生和发展中具有一定的意义。同时, AIS的炎症改变以T细胞浸润为主要表现, 炎症细胞浸润、MHC-1表达与抗肌萎缩蛋白异常表达之间似有一定的相关性。沿此结果进一步的研究可能为AIS的诊断和针对椎旁肌病变的治疗开辟新的思路。
中图分类号:
- R682.3
| 1 |
Cheng JC , Castelein RM , Chu WC , et al. Adolescent idiopathic scoliosis[J]. Nat Rev Dis Primers, 2015, 1, 15030.
doi: 10.1038/nrdp.2015.30 |
| 2 | Weinstein SL . The natural history of adolescent idiopathic scoliosis[J]. J Pediatr Orthop, 2019, 39 (6): S44- S46. |
| 3 |
Spencer GS , Eccles MJ . Spinal muscle in scoliosis: Part 2. The proportion and size of type 1 and type 2 skeletal muscle fibres measured using a computer-controlled microscope[J]. J Neurol Sci, 1976, 30 (1): 143- 154.
doi: 10.1016/0022-510X(76)90262-8 |
| 4 | Fidler MW , Jowett RL . Muscle imbalance in the aetiology of sco-liosis[J]. J Bone Joint Surg B, 1976, 58 (2): 200- 201. |
| 5 |
Khosla S , Tredwell SJ , Day B , et al. An ultrastructural study of multifidus muscle in progressive idiopathic scoliosis. Changes resulting from a sarcolemmal defect at the myotendinous junction[J]. J Neurol Sci, 1980, 46 (1): 13- 31.
doi: 10.1016/0022-510X(80)90040-4 |
| 6 |
Tidball JG . Myotendinous junction: Morphological changes and mechanical failure associated with muscle cell atrophy[J]. Exp Mol Pathol, 1984, 40 (1): 1- 12.
doi: 10.1016/0014-4800(84)90060-1 |
| 7 |
Wajchenberg M , Martins DE , Luciano RP , et al. Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: A cross-sectional study[J]. Medicine (Baltimore), 2015, 94 (8): e598.
doi: 10.1097/MD.0000000000000598 |
| 8 |
Luciano Rde P , Puertas EB , Martins DE , et al. Adolescent idiopathic scoliosis without limb weakness: A differential diagnosis of core myopathy?[J]. BMC Musculoskelet Disord, 2015, 16, 179.
doi: 10.1186/s12891-015-0629-8 |
| 9 |
Zoabli G , Mathieu PA , Aubin CE . Magnetic resonance imaging of the erector spinae muscles in Duchenne muscular dystrophy: Implication for scoliotic deformities[J]. Scoliosis, 2008, 3, 21.
doi: 10.1186/1748-7161-3-21 |
| 10 |
Nash CL Jr , Moe JH . A study of vertebral rotation[J]. J Bone Joint Surg Am, 1969, 51 (2): 223- 229.
doi: 10.2106/00004623-196951020-00002 |
| 11 | Dubowitz V , Sewry CA , Oldfors A , et al. Muscle biopsy: A practical approach[M]. 4th ed. Oxford, England: Saunders Else-vier, 2013. |
| 12 |
Altaf F , Gibson A , Dannawi Z , et al. Adolescent idiopathic sco-liosis[J]. BMJ, 2013, 346, f2508.
doi: 10.1136/bmj.f2508 |
| 13 |
Brzoska E , Kalkowski L , Kowalski K , et al. Muscular contribution to adolescent idiopathic scoliosis from the perspective of stem cell-based regenerative medicine[J]. Stem Cells Dev, 2019, 28 (16): 1059- 1077.
doi: 10.1089/scd.2019.0073 |
| 14 |
Veldhuizen AG , Wever DJ , Webb PJ . The aetiology of idiopathic scoliosis: Biomechanical and neuromuscular factors[J]. Eur Spine J, 2000, 9 (3): 178- 184.
doi: 10.1007/s005860000142 |
| 15 |
Meier MP , Klein MP , Krebs D , et al. Fiber transformations in multifidus muscle of young patients with idiopathic scoliosis[J]. Spine (Phila Pa 1976), 1997, 22 (20): 2357- 2364.
doi: 10.1097/00007632-199710150-00008 |
| 16 | Bylund P , Jansson E , Dahlberg E , et al. Muscle fiber types in thoracic erector spinae muscles[J]. Clin Orthop Relat Res, 1987, (214): 222- 228. |
| 17 |
Mannion AF , Meier M , Grob D , et al. Paraspinal muscle fibre type alterations associated with scoliosis: an old problem revisited with new evidence[J]. Eur Spine J, 1998, 7 (4): 289- 293.
doi: 10.1007/s005860050077 |
| 18 |
Hoffman EP , Brown RH Jr , Kunkel LM . Dystrophin: The protein product of the Duchenne muscular dystrophy locus[J]. Cell, 1987, 51 (6): 919- 928.
doi: 10.1016/0092-8674(87)90579-4 |
| 19 |
Ervasti JM , Campbell KP . Membrane organization of the dystrophin-glycoprotein complex[J]. Cell, 1991, 66 (6): 1121- 1231.
doi: 10.1016/0092-8674(91)90035-W |
| 20 |
Houang EM , Sham YY , Bates FS , et al. Muscle membrane integrity in Duchenne muscular dystrophy: Recent advances in copolymer-based muscle membrane stabilizers[J]. Skelet Muscle, 2018, 8 (1): 31.
doi: 10.1186/s13395-018-0177-7 |
| 21 |
Yiu EM , Kornberg AJ . Duchenne muscular dystrophy[J]. J Paediatr Child Health, 2015, 51 (8): 759- 764.
doi: 10.1111/jpc.12868 |
| 22 |
Wilson K , Faelan C , Patterson-Kane JC , et al. Duchenne and Becker muscular dystrophies: A review of animal models, clinical end points, and biomarker quantification[J]. Toxicol Pathol, 2017, 45 (7): 961- 976.
doi: 10.1177/0192623317734823 |
| 23 | Goebel HH , Sewry CA , Weller RO . Muscle disease: Pathology and genetics[M]. 2nd ed. New Jersey, USA: Wiley-Blackwell, 2013: 95- 100. |
| 24 | Gherardi RK . Pathogenic aspects of dermatomyositis, polymyositis and overlap myositis[J]. Presse Med, 2011, 40 (4 Pt 2): e209- e218. |
| 25 |
Samaan MC , Missiuna P , Peterson D , et al. Understanding the role of the immune system in adolescent idiopathic scoliosis: Immunometabolic CONnections to Scoliosis (ICONS) study protocol[J]. BMJ Open, 2016, 6 (7): e011812.
doi: 10.1136/bmjopen-2016-011812 |
| 26 |
Rudrapatna S , Peterson D , Missiuna P , et al. Understanding muscle-immune interactions in adolescent idiopathic scoliosis: A feasibility study[J]. Pilot Feasibility Stud, 2017, 3, 50.
doi: 10.1186/s40814-017-0193-0 |
| 27 |
Van Gennip JLM , Boswell CW , Ciruna B . Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis[J]. Sci Adv, 2018, 4 (12): eaav1781.
doi: 10.1126/sciadv.aav1781 |
| 28 |
Dalkilic I , Kunkel LM . Muscular dystrophies: Genes to pathoge-nesis[J]. Curr Opin Genet Dev, 2003, 13 (3): 231- 238.
doi: 10.1016/S0959-437X(03)00048-0 |
| 29 |
Fiorillo AA , Heier CR , Novak JS , et al. TNF-α-induced micro-RNAs control dystrophin expression in Becker muscular dystrophy[J]. Cell Rep, 2015, 12 (10): 1678- 1690.
doi: 10.1016/j.celrep.2015.07.066 |
| [1] | 王敏, 李倩. 青少年抑郁症患者心理弹性影响因素的路径分析[J]. 北京大学学报(医学版), 2024, 56(5): 809-814. |
| [2] | 李志存, 吴天俣, 梁磊, 范宇, 孟一森, 张骞. 穿刺活检单针阳性前列腺癌术后病理升级的危险因素分析及列线图模型构建[J]. 北京大学学报(医学版), 2024, 56(5): 896-901. |
| [3] | 田宇轩,阮明健,刘毅,李德润,吴静云,沈棋,范宇,金杰. 双参数MRI改良PI-RADS评分4分和5分病灶的最大径对临床有意义前列腺癌的预测效果[J]. 北京大学学报(医学版), 2024, 56(4): 567-574. |
| [4] | 姚凯烽,阮明健,李德润,田宇轩,陈宇珂,范宇,刘毅. 靶向穿刺联合区域系统穿刺对PI-RADS 4~5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2024, 56(4): 575-581. |
| [5] | 沈鹤军,侍崇艳,郑清,黄玉,景涛. 我国高中生静坐时长与健康素养现状及其影响因素调查[J]. 北京大学学报(医学版), 2024, 56(2): 239-246. |
| [6] | 刘毅,袁昌巍,吴静云,沈棋,肖江喜,赵峥,王霄英,李学松,何志嵩,周利群. 靶向穿刺+6针系统穿刺对PI-RADS 5分患者的前列腺癌诊断效能[J]. 北京大学学报(医学版), 2023, 55(5): 812-817. |
| [7] | 袁昌巍,李德润,李志华,刘毅,山刚志,李学松,周利群. 多参数磁共振成像中动态对比增强状态在诊断PI-RADS 4分前列腺癌中的应用[J]. 北京大学学报(医学版), 2023, 55(5): 838-842. |
| [8] | 田聪,刘军,杨波,乔佳佳,黄晓波,许清泉. 经皮肾镜取石术中异常肾盂黏膜活检结果分析[J]. 北京大学学报(医学版), 2023, 55(5): 948-952. |
| [9] | 崔孟杰,马奇,陈曼曼,马涛,王鑫鑫,刘婕妤,张奕,陈力,蒋家诺,袁雯,郭桐君,董彦会,马军,星一. 不同生长模式与7~17岁儿童青少年代谢综合征的关系[J]. 北京大学学报(医学版), 2023, 55(3): 415-420. |
| [10] | 党佳佳,蔡珊,钟盼亮,王雅琪,刘云飞,师嫡,陈子玥,张依航,胡佩瑾,李晶,马军,宋逸. 室外夜间人工光暴露与中国9~18岁儿童青少年超重肥胖的关联[J]. 北京大学学报(医学版), 2023, 55(3): 421-428. |
| [11] | 刘云飞,党佳佳,钟盼亮,马宁,师嫡,宋逸. 1990—2019年中国5~24岁人群伤害死亡率分析[J]. 北京大学学报(医学版), 2022, 54(3): 498-504. |
| [12] | 陈曼曼,杨招庚,苏彬彬,李艳辉,高迪,马莹,马涛,董彦会,马军. 中山市儿童青少年青春期身高生长突增规律[J]. 北京大学学报(医学版), 2021, 53(3): 506-510. |
| [13] | 杨雪,孙伟,王哲,姬爱平,白洁. 儿童和青少年牙外伤急诊患者临床分析[J]. 北京大学学报(医学版), 2021, 53(2): 384-389. |
| [14] | 周境,刘怡. 不同垂直骨面型骨性Ⅱ类青少年女性颞下颌关节锥形束CT测量分析[J]. 北京大学学报(医学版), 2021, 53(1): 109-119. |
| [15] | 郝一昌,颜野,张帆,邱敏,周朗,刘可,卢剑,肖春雷,黄毅,刘承,马潞林. 穿刺活检单针阳性的前列腺癌手术策略选择及经验总结[J]. 北京大学学报(医学版), 2020, 52(4): 625-631. |
|
||