北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (5): 957-963. doi: 10.19723/j.issn.1671-167X.2021.05.025
远端型遗传性运动神经病8例的临床、病理及遗传学特点
刘梅歌1,方朴2,王严1,丛璐1,范洋溢1,袁远1,徐燕1,张俊1,洪道俊1,2,△( )
)
- 1.北京大学人民医院神经内科,北京 100044
2.南昌大学第一附属医院神经内科,南昌 330006
Clinical, pathological and genetic characteristics of 8 patients with distal hereditary motor neuropathy
LIU Mei-ge1,FANG Pu2,WANG Yan1,CONG Lu1,FAN Yang-yi1,YUAN Yuan1,XU Yan1,ZHANG Jun1,HONG Dao-jun1,2,△( )
)
- 1. Department of Neurology, Peking University People’s Hospital, Beijing 100044, China
2. Department of Neurology, the First Affiliated Hospital of Nanchang University, Nanchang 330006, China
摘要:
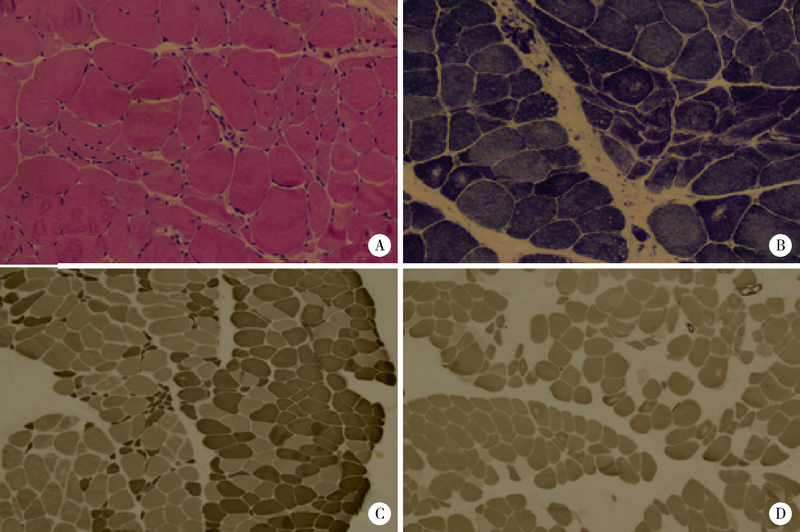
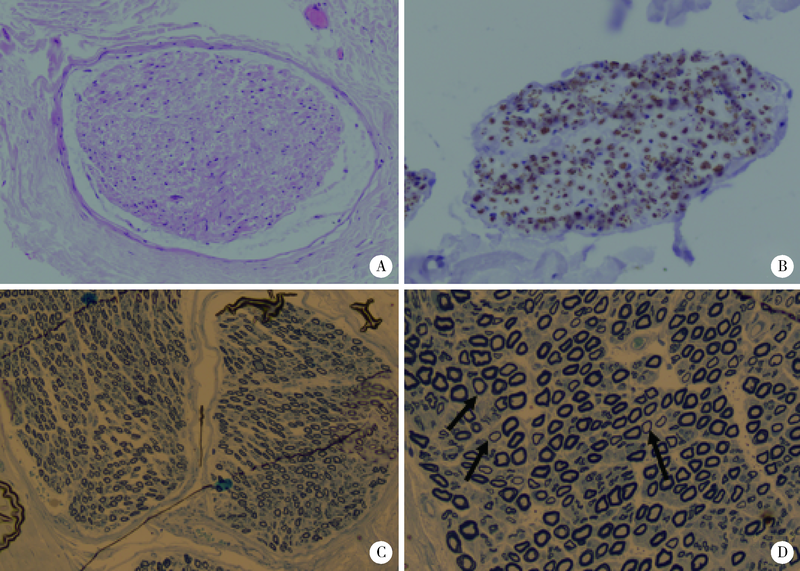
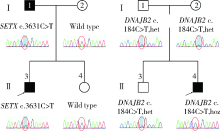
目的: 远端型遗传性运动神经病(distal hereditary motor neuropathy, dHMN)是一组选择性累及运动神经及其神经元的退行性病变,可引起肢体远端肌肉进行性萎缩无力。总结8例dHMN先证者的临床、电生理、病理及遗传学特点,丰富我国dHMN先证者的临床表型和基因型资料,提高临床工作者对dHMN的认识和诊治水平。方法: 选择2018年6月至2019年4月于北京大学人民医院神经内科就诊的8例dHMN先证者并进而追踪其家系,回顾性分析先证者的临床症状、神经电生理改变、病理特点及基因突变情况。运用基因靶向二代测序技术对所有先证者进行周围神经病相关基因检测,通过 Sanger测序验证突变位点,并对可获得的家系成员进行遗传共分离分析。结果: 先证者发病年龄11~64岁,中位数39.5岁,均为慢性起病,进行性发展,主要表现为远端肢体无力,并逐渐出现肌肉萎缩。神经电生理结果示选择性运动神经损害,运动神经复合肌肉动作电位波幅下降伴神经传导速度减慢,感觉神经不受累,针刺肌电图符合神经源性损害表现。2例先证者肌肉活检显示神经源性骨骼肌损害,1例先证者腓肠神经活检提示感觉神经受累轻微。基因测序显示8例先证者携带了8种不同的已知dHMN致病基因,3例有已报道的致病突变位点,基因诊断率为37.5%,其余5例为临床意义未明的新发点突变,其中2例突变在家系内共分离。结论: dHMN是一组临床和基因均具有显著异质性的遗传性周围神经病,二代测序技术广泛运用于dHMN先证者的致病基因搜寻,但仍有超过一半的先证者不能得到明确的基因诊断。
中图分类号:
- R596
| [1] |
Garg N, Park SB, Vucic S, et al. Differentiating lower motor neuron syndrome [J]. J Neurol Neurosurg Psychiatry, 2017, 88(6):474-483.
doi: 10.1136/jnnp-2016-313526 |
| [2] |
Frasquet M, Rojas-García R, Argente-Escrig H, et al. Distal hereditary motor neuropathies: mutation spectrum and genotype-phenotype correlation [J]. Eur J Neurol, 2021, 28(4):1334-1343.
doi: 10.1111/ene.14700 pmid: 33369814 |
| [3] |
Bansagi B, Griffin H, Whittaker RG, et al. Genetic heterogeneity of motor neuropathies [J]. Neurology, 2017, 88(13):1226-1234.
doi: 10.1212/WNL.0000000000003772 |
| [4] | Bacquet J, Stojkovic T, Boyer A, et al. Molecular diagnosis of inherited peripheral neuropathies by targeted next-generation sequencing: molecular spectrum delineation [J]. BMJ Open, 2018, 8(10):e21632. |
| [5] |
Echaniz-Laguna A, Geuens T, Petiot P, et al. Axonal neuropathies due to mutations in small heat shock proteins: clinical, genetic, and functional insights into novel mutations [J]. Hum Mutat, 2017, 38(5):556-568.
doi: 10.1002/humu.23189 pmid: 28144995 |
| [6] |
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [J]. Genet Med, 2015, 17(5):405-424.
doi: 10.1038/gim.2015.30 pmid: 25741868 |
| [7] |
Tanabe H, Higuchi Y, Yuan JH, et al. Clinical and genetic features of charcot-marie-tooth disease 2F and hereditary motor neuropathy 2B in Japan [J]. J Peripher Nerv Syst, 2018, 23(1):40-48.
doi: 10.1111/jns.2018.23.issue-1 |
| [8] |
Windpassinger C, Auer-Grumbach M, Irobi J, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome [J]. Nat Genet, 2004, 36(3):271-276.
doi: 10.1038/ng1313 |
| [9] |
Novarino G, Fenstermaker AG, Zaki MS, et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders [J]. Science, 2014, 343(6170):506-511.
doi: 10.1126/science.1247363 pmid: 24482476 |
| [10] | Xie Y, Lin Z, Pakhrin PS, et al. Genetic and clinical features in 24 Chinese distal hereditary motor neuropathy families [J/OL]. Front Neurol, 2020, 11:603003(2020-12-14)[2020-12-15]. https://pubmed-ncbi-nlm-nih-gov-443.webvpn.bjmu.edu.cn/33 3810781/ . |
| [11] | 张付峰, 卢晓琴, 严新翔, 等. 远端型遗传性运动神经病的临床特征分析 [J]. 第二军医大学学报, 2009, 30(1):57-60. |
| [12] |
De Jonghe P, Auer-Grumbach M, Irobi J, et al. Autosomal dominant juvenile amyotrophic lateral sclerosis and distal hereditary motor neuronopathy with pyramidal tract signs: synonyms for the same disorder [J]. Brain, 2002, 125(Pt 6):1320-1325.
pmid: 12023320 |
| [13] |
Motley WW, Griffin LB, Mademan I, et al. A novel AARS mutation in a family with dominant myeloneuropathy [J]. Neurology, 2015, 84(20):2040-2047.
doi: 10.1212/WNL.0000000000001583 |
| [14] |
Luigetti M, Fabrizi GM, Madia F, et al. Seipin S90L mutation in an Italian family with CMT2/dHMN and pyramidal signs [J]. Muscle Nerve, 2010, 42(3):448-451.
doi: 10.1002/mus.21734 pmid: 20806400 |
| [15] |
Beecroft SJ, McLean CA, Delatycki MB, et al. Expanding the phenotypic spectrum associated with mutations of DYNC1H1 [J]. Neuromuscul Disord, 2017, 27(7):607-615.
doi: 10.1016/j.nmd.2017.04.011 |
| [16] |
Dierick I, Baets J, Irobi J, et al. Relative contribution of mutations in genes for autosomal dominant distal hereditary motor neuropathies: a genotype-phenotype correlation study [J]. Brain, 2008, 131(Pt 5):1217-1227.
doi: 10.1093/brain/awn029 pmid: 18325928 |
| [17] |
Rossor AM, Evans MR, Reilly MM. A practical approach to the genetic neuropathies [J]. Pract Neurol, 2015, 15(3):187-198.
doi: 10.1136/practneurol-2015-001095 |
| [18] |
Liu X, Duan X, Zhang Y, Sun A, et al. Molecular analysis and clinical diversity of distal hereditary motor neuropathy [J]. Eur J Neurol, 2020, 27:1319-1326.
doi: 10.1111/ene.14260 pmid: 32298515 |
| [19] |
Beijer D, Baets J. The expanding genetic landscape of hereditary motor neuropathies [J]. Brain, 2020, 143(Pt 12):3540-3563.
doi: 10.1093/brain/awaa311 |
| [1] | 焦莶如, 龚潘, 牛悦, 徐兆, 周宗朴, 杨志仙. 以婴儿癫痫性痉挛综合征为表型的吡哆醇依赖性癫痫[J]. 北京大学学报(医学版), 2024, 56(5): 781-787. |
| [2] | 武志慧, 胡明智, 赵巧英, 吕凤凤, 张晶莹, 张伟, 王永福, 孙晓林, 王慧. miR-125b-5p修饰脐带间充质干细胞对系统性红斑狼疮的免疫调控机制[J]. 北京大学学报(医学版), 2024, 56(5): 860-867. |
| [3] | 刘东武, 陈杰, 高明利, 于静. 类风湿关节炎伴发淋巴结Castleman样病理改变1例[J]. 北京大学学报(医学版), 2024, 56(5): 928-931. |
| [4] | 刘帅,刘磊,刘茁,张帆,马潞林,田晓军,侯小飞,王国良,赵磊,张树栋. 伴静脉癌栓的肾上腺皮质癌的临床治疗及预后[J]. 北京大学学报(医学版), 2024, 56(4): 624-630. |
| [5] | 郭煌达,彭和香,王斯悦,侯天姣,李奕昕,章涵宇,王梦莹,武轶群,秦雪英,唐迅,李劲,陈大方,胡永华,吴涛. 短期大气颗粒物暴露和MTNR1B基因多态性对甘油三酯-葡萄糖指数影响的家系研究[J]. 北京大学学报(医学版), 2024, 56(3): 375-383. |
| [6] | 侯天姣,周治波,王竹青,王梦莹,王斯悦,彭和香,郭煌达,李奕昕,章涵宇,秦雪英,武轶群,郑鸿尘,李静,吴涛,朱洪平. 转化生长因子β信号通路与非综合征型唇腭裂发病风险的基因-基因及基因-环境交互作用[J]. 北京大学学报(医学版), 2024, 56(3): 384-389. |
| [7] | 王鹏,杨子瑶,王萌,王巍,李爱芝. 2例罕见RhD变异型RHD*DEL37的分子生物学分析[J]. 北京大学学报(医学版), 2024, 56(2): 352-356. |
| [8] | 刘欢锐,彭祥,李森林,苟欣. 基于HER-2相关基因构建风险模型用于膀胱癌生存预后评估[J]. 北京大学学报(医学版), 2023, 55(5): 793-801. |
| [9] | 薛子璇,唐世英,邱敏,刘承,田晓军,陆敏,董靖晗,马潞林,张树栋. 青年肾肿瘤伴瘤栓的临床病理特征及预后分析[J]. 北京大学学报(医学版), 2023, 55(5): 802-811. |
| [10] | 金银姬,孙琳,赵金霞,刘湘源. 血清IgA型抗鼠科肉瘤病毒癌基因同源物B1抗体在类风湿关节炎中的意义[J]. 北京大学学报(医学版), 2023, 55(4): 631-635. |
| [11] | 谢尚,蔡志刚,单小峰. 全外显子测序及相关指标在口腔鳞状细胞癌精准治疗中的应用价值[J]. 北京大学学报(医学版), 2023, 55(4): 697-701. |
| [12] | 许媛媛,孙志琳,张秀莲,刘子莲,刘维,关欣. 卡马西平致HLA-A * 3101基因阳性中国汉族人发生Stevens-Johnson综合征1例[J]. 北京大学学报(医学版), 2023, 55(4): 755-757. |
| [13] | 史佳琪,马莺,张奕,陈章健,贾光. 纳米二氧化钛颗粒对人肝癌细胞HepG2中circRNA表达谱的影响[J]. 北京大学学报(医学版), 2023, 55(3): 392-399. |
| [14] | 王雪珩,王斯悦,彭和香,范梦,郭煌达,侯天姣,王梦莹,武轶群,秦雪英,唐迅,李劲,陈大方,胡永华,吴涛. 基因-环境交互作用对动脉僵硬度影响的家系研究[J]. 北京大学学报(医学版), 2023, 55(3): 400-407. |
| [15] | 时云飞,王豪杰,刘卫平,米岚,龙孟平,刘雁飞,赖玉梅,周立新,刁新婷,李向红. 血管免疫母细胞性T细胞淋巴瘤临床与分子病理学特征分析[J]. 北京大学学报(医学版), 2023, 55(3): 521-529. |
|
||